Cadila Healthcare Ltd
Company Overview
The company was founded in the year 1952 by Mr. Ramanbhai B. Patel (late), a first-generation entrepreneur and a doyen in the field of Indian Pharmaceuticals. 1
In 1995, the group was restructured and thus was formed Cadila Healthcare (NSE: CADILAHC) under the aegis of the Zydus group. From a humble turnover Rs. 250 crores in 1995 the group witnessed a significant financial growth and registered a turnover of over Rs. 14,253 crores in FY20.
Headquartered in Ahmedabad, India, the group ranks 4th in the Indian pharmaceutical industry with manufacturing sites and research facilities spread across five states of Gujarat, Maharashtra, Goa, Himachal Pradesh and Sikkim. Globally, the group has a strong presence in the regulated markets of the US, Europe (France & Spain) and the high profile markets of Latin America and South Africa. It also has a strong presence in 25 other emerging markets worldwide.

India Business
The India Business comprises the India formulations business spearheaded by Zydus Healthcare Ltd., based in Mumbai and the Biologics business which drives the sales and marketing of biosimilars and novel biologics.
Zydus Healthcare Ltd.
Zydus is one of the oldest players in the Indian formulations market and has gone onto become a prominent pharmaceutical manufacturer in India. Besides continuously improving its market presence and market share, the group has also expanded its portfolio by entering newer therapeutic areas.The group has been launching new products with the first mover advantage and has a strong presence in both acute and chronic therapies.
These strategic initiatives have helped Zydus become one of the dominant players in the Indian formulations market with the leadership position in several therapeutic categories.The group’s innovative NCE therapy Lipaglyn, a novel drug to be approved for the treatment of diabetic dyslipidemia is marketed by Zydus Discovery, a division of Zydus Healthcare Ltd.
Zydus Biologics
Zydus Biologics, a super-specialty centric business entity of Cadila healthcare Ltd., is engaged in manufacturing and commercialization of both, small molecule, biological drugs and vaccines intended for prevention and treatment of various diseases in the field of oncology, hepatology, nephrology, rheumatology, ophthalmology and bone health. With capabilities across the value chain - Drug development, manufacturing, distribution and commercialization, Zydus Biologics has emerged as one of the leading players in the biologics market and has a formidable presence in its participatory market. Zydus Biologics' developmental capabilities cover fusion proteins, monoclonal antibodies, antibody drug conjugates and vaccines. It has a robust pipeline comprising of next generation biosimilars, and novel biologicals.
As part of its vision of being at the forefront of innovation led growth, Zydus Biologics has undertaken several pioneering initiatives like launching Exemptia, world's first biosimilar of Adalimumab, Vaxiflu S - Indian's first indigenously developed H1N1 vaccine for protection against swine flu, Vaxiflu 4 - India's first quadrivalent influenza vaccine and ZyVac TCV – World’s second brand of typhoid conjugate vaccine.
Zydus Animal Health
Zydus Animal Health, a division of the group is India’s leading animal healthcare player and a market leader in various therapeutic segments which include anti-bacterial, NSAIDs, Anti-mastitis, tonics and poultry vaccines amongst others in India. With a strong presence in the livestock and poultry segments, Zydus Animal Health has also launched a basket of products specifically for the companion animals to cater the increasing demands of the pet community.
US Formulations Business
Ranked 4th among US generic companies based on prescriptions with an increase in market share by 0.62% which is a gain of three positions from last year.
Launch 30 new products during the year. New launches include Rivastigmine Transdermal Patch, which is the first transdermal patch launched from the Company’s own pipeline.
Filed 30 additional ANDAs with the USFDA during the year, taking the cumulative number of filings to 390.
Emerging Markets of Asia, Africa And Latin America
Continued to perform well in some of the key geographies of Asia Pacific and Africa region with double digit growth registered in several countries driven by volume expansion of pillar brands.
Manufacturing Capabilities
The group has manufacturing capabilities across the value chain including formulations, APIs, vaccines, biosimilars, complex products (transdermals, topical etc.), animal health products as well as wellness products with more than 30 manufacturing plants worldwide including India, Germany, Brazil & USA
State-of-the-art Plants
- Finished Dosage Form Moraiya Ahmedabad
- Finished Dosage Form, Baddi
- Finished Dosage Form Goa
- Formulations Plant at Sikkim
- API-Dabhasa
- API, Ahmedabad
- Biologics Active Substances, Zydus Biologics, Ahmedabad
- Alidac Pharmaceuticals Ltd., Pharmez, Ahmedabad
- Cytotoxic Injectable JV with Pfizer
- VaccineTechnologyCentre, Ahmedabad
- Transdermals Manufacturing Zydus Technologies Limited, Pharmez, Ahmedabad
- Topical Formulation Manufacturing, Pharmez, Ahmedabad
- Finished Dosage Form, Brazil
- Liquid & Solid Oral Dosage Products, Nesher Pharma, USA
Financial Overview
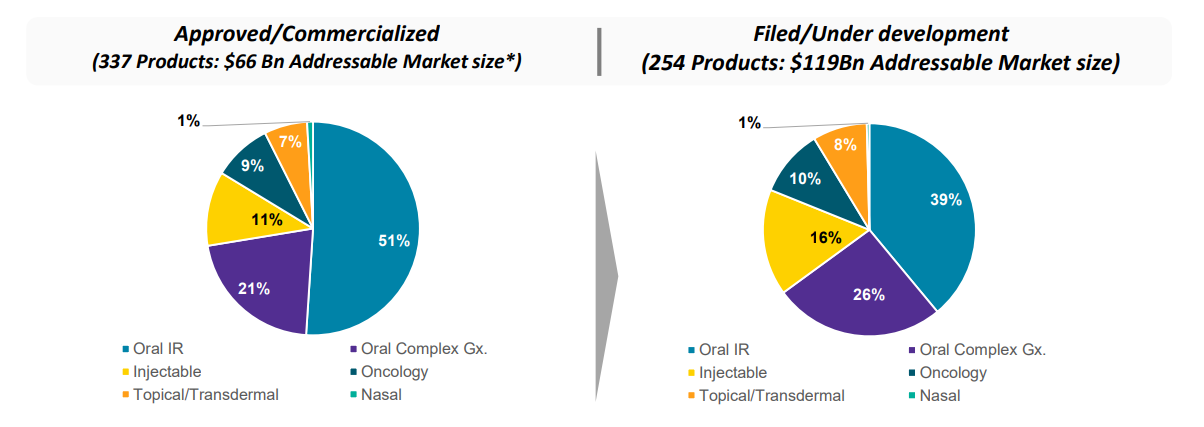
During the year ended March 31, 2020, the consolidated revenue from operations and other income was Rs 143,670 Million. The Company has achieved consolidated Profit Before Tax of Rs 14,954 Million and Profit After Tax of ` 12,044 Million. The Company achieved a consolidated total Comprehensive Income of Rs 9,039 Million. The EPS on consolidated financials for the year ended on March 31, 2020 was Rs 11.49. 2
Subsidiary Companies
The Company has incorporated a new company in the name of Alidac Healthcare Limited (“AHL”), as a wholly owned subsidiary company, for manufacturing of human formulations in Special Economic Zone (“Pharmez”), Matoda, Ahmedabad. Later on, the name of AHL was changed to Zydus Pharmaceuticals Limited.
A partnership firm was formed in the name of Recon Pharmaceuticals and Investments, in which Zydus Healthcare Limited (“ZHL”) and German Remedies Pharmaceuticals Private Limited (formerly known as Acme Pharmaceuticals Private Limited) (“GRPPL”) are 90% and 10% partners respectively. The said partnership firm carries on business of trading of pharmaceuticals and holding investments.
The Company acquired 15% equity share capital of Zydus Technologies Limited (“ZTL”), in which the Company was already holding 85% equity share capital. In view of the same, ZTL became a wholly owned subsidiary of the Company.
The company acquired 15% common stock of Zydus Noveltech Inc., USA (“ZNI”), in which the Company was already holding 85% common stock. In view of the same, ZNI became a wholly owned subsidiary of the Company.
Scheme of Amalgamation of Zydus Technologies Limited (“ZTL”), Alidac Pharmaceuticals Limited (“APL”), Liva Pharmaceuticals Limited (“LPL”) and Dialforhealth India Limited (“DHIL”) with Cadila Healthcare Limited (“CHL” or “the Company”) (“Scheme 1”) was sanctioned by the Hon’ble National Company Law Tribunal, Bench at Ahmedabad (“NCLT”), vide its Order dated March 16, 2020. The Scheme was made effective from March 31, 2020. The Appointed Date for the Scheme 1 is April 1, 2019.
Recent developments
Zydus Cadila consolidated profits jump 73% in Q2 3
For the second quarter ended September 30, 2020, Zydus Cadila reported total income from operations of Rs. 3820 crores up by 13% on a y-o-y basis. Consolidated EBIDTA grew to Rs. 863 crores, up 36% on y-o-y basis. The EBIDTA margins stood at 22.6% during the quarter, which improved significantly by 370 basis points compared to 18.9% registered in Q2 of FY 20. Consolidated Profit After Tax excl. exceptional items for the quarter was Rs. 562 crores, up 73% ona y-o-y basis and up 24% on a sequential basis.
Strengthening its financial position, the Company has significantly reduced its net debt-by Rs. 2709 crores in the first six months of FY21, which is 40% reduction from net debt reported in March 2020., The net debt as on 30th September, 2020 stood at Rs. 4031 crores against Rs. 6740 crores as on 31st March, 2020.
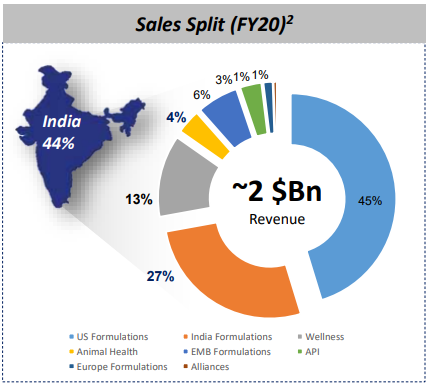
The company’s India business which comprises human health, consumer wellness and animal health business posted sales of Rs.1583 crores up by 11% on a y-o-y basis. The Company gained market share in Gynaecology, Pain management, Anti-Infectives, Anti-Diabetic and Hormones portfolio during the quarter as compared to the corresponding quarter of the previous financial year. The animal health business in India saw a significant improvement in the performance during the quarter as the business posted a sales of Rs. 161 crores during the quarter, with a growth of 20% on a y-o-y basis. The growth was driven by good demand and the strong equity that our brands have in the market. The ° company’s business in the US posted sales of Rs. 1709 crores up by 18% on a y-o-y basis. During the quarter, the company launched 6 new products in the US. The Company received approval for 10 new products (incl. 2 tentative approvals) and filed 5 additional ANDAs with the USFDA during the quarter. The company’s business in the emerging markets of Asia, Africa and Latin America grew by 12% in constant currency.
During the quarter, the Company received the final approval from the U.S. Food and Drug Administration (FDA) for its Abbreviated New Drug Applications (ANDAs) for Liposomal Doxorubicin injection. With this, Zydus became the first generic company to perform whole array of product development-including the current stringent US FDA requirements, manufacturing, and commercialization through its own capabilities.
Making progress with its research initiatives to fight COVID 19, the Company will be completing the pre-clinical development on ZYIL 1, a small molecule NCE positioned for management of critically ill COVID 19 patients. The Phase II clinical trials of Desidustat in the management of COVID 19 is underway at Mexico. The Company has also completed Phase II clinical trials of Pegylated Interferon Alpha-2b in India for management of COVID 19. The Company has launched Remdesivir injection in India and Emerging markets at the most economical price providing greater access to the therapy by making it more affordable to patients.
The Adaptive Phase I/II clinical trials are underway for the Company’s lead vaccine candidate ZyCoV-D. The plasmid DNA platform on which our vaccine is based also provides ease of manufacturing with minimal biosafety requirements (BSL-1). The platform is also known to show much improved vaccine stability and lower cold chain requirements making it easy for transportation to the remotest regions of the country.




