Ritter Pharmaceuticals
Ritter Pharmaceuticals, Inc. (RTTR) develops novel therapeutic products that modulate the human gut microbiome to treat gastrointestinal diseases. Ritter Pharmaceuticals is advancing human gut health research by exploring the metabolic capacity of the gut microbiota and translating the functionality of prebiotic-based therapeutics into applications intended to have a meaningful impact on a patient’s health. The company completed a Phase 2a clinical trial of its leading product candidate, RP-G28, an orally administered, high purity oligosaccharide in November 2011.1
The company completed a Phase 2b/3 multi-center, randomized, double-blind, placebo-controlled, parallel group trial of RP-G28 in October 2016. The purpose of the trial was to evaluate the safety, efficacy and tolerability of two dosing regimens of RP-G28 in patients with moderate to severe lactose intolerance symptoms. Enrollment was initiated in March 2016 and completed in August 2016, achieving its projected enrollment time period. The trial aimed to evaluate a patient’s ability to consume dairy foods post-treatment with improved tolerance and reduced digestive symptoms. A total of 377 subjects were enrolled in the trial with 18 clinical sites participating throughout the United States. Patients underwent a 30-day treatment, followed by a 30-day post-treatment evaluation of dairy tolerance. On October 17, 2016, the last patient completed dosing and all monitoring visits.
The company held a Type C meeting with the FDA in March 2017, prior to the unblinding of its Phase 2b/3 data, to discuss its development plans and Phase 2b/3 clinical trial. The focus of the meeting was to obtain the FDA’s feedback on its Phase 2b/3 clinical trial, including its statistical analysis plan (“SAP”) prior to unblinding any data.
The meeting with the FDA was constructive and productively focused on best defining clinically meaningful benefits to patients suffering from lactose intolerance and how to reflect these benefits in endpoints. The company modified aspects of its SAP to address certain FDA recommendations, including a change to its primary endpoint, which was changed to combine abdominal pain with relevant secondary endpoints to establish a composite score (abdominal pain, abdominal cramping, abdominal bloating and abdominal gas). The protocol design and the assessment utilized to collect lactose intolerance symptoms remained unchanged.
Topline results of the trial were announced in March 2017. Due to inconsistent data results from one study site, the data from this site was excluded from the primary analysis population (Efficacy Subset mITT). After excluding the data from the one anomalous study site, results showed a clinically meaningful benefit to subjects in the reduction of lactose intolerance symptoms across a variety of outcome measures. The majority of analyses showed positive outcome measures and the robustness of the data point to a clear drug effect. Treatment patients not only reported meaningful reduced symptoms, but also 30 days after taking the treatment, patients reported adequate relief from lactose intolerance symptoms and satisfaction with the results of the treatment, with RP-G28 preventing or treating their lactose intolerance symptoms. Greater milk and dairy product consumption was also reported by patients.
A subset of subjects from its Phase 2b/3 clinical trial has been rolled into a 12-month extension study to evaluate long-term durability of treatment. The study is also evaluating each participant’s microbiome, expanding its knowledge of the effects that RP-G28 may have on adapting the gut microbiota in a beneficial manner. The subjects are expected to complete the 12-month evaluation during the fourth quarter of 2017.
The company held an End-of-Phase 2 meeting with the FDA’s Division of Gastroenterology and Inborn Errors Products in August 2017. The purpose of the meeting was to obtain the FDA’s feedback on its Phase 3 program. The company reached general consensus with the FDA on certain elements of its current Phase 3 program and have received clear guidance and recommendations on many necessary components of its Phase 3 program; including the clinical, non-clinical, and chemistry, manufacturing and controls (CMC) requirements needed to support an NDA submission.
Ritter Pharmaceuticals has incorporated much of this guidance into its Phase 3 program. The company's current Phase 3 clinical program will consist of two confirmatory clinical trials of similar trial design and size as its Phase 2b/3 clinical trial and will include additional components that may allow for claims for durability of effect. These additional trials may be run in parallel.
Leading Product Candidate — RP-G28
RP-G28 is a novel highly purified GOS, which is synthesized enzymatically. The product is being developed to reduce the symptoms and frequency of episodes of abdominal pain associated with lactose intolerance. The therapeutic is taken orally (a powder solution mixed in water) for 30 consecutive days. The proposed mechanism of action of RP-G28 is to increase the intestinal growth and colonization of bacteria that can metabolize lactose to compensate for a patient’s intrinsic inability to digest lactose. Once colonization of bacteria has occurred, it is hypothesized that patients will continue to tolerate lactose as long as they maintain their microflora balance. RP-G28 has the potential to become the first FDA-approved drug for the reduction of symptoms associated with lactose intolerance.
Galactooligosaccharides (GOS)
RP-G28 is a >95% purified GOS product. GOS refers to a group of compounds containing β-linkages of 1 to 6 galactose units with a single glucose on the terminal end and are found at low levels in human milk. GOS products resist hydrolysis by salivary and intestinal enzymes because of the configuration of their glycosidic bonds and reach the colon virtually intact. The undigested GOS enhances the growth of beneficial, lactose metabolizing, colonic bacteria that already exist in the subject’s digestive track, including multiple species and strains of bifidobacteria and lactobacilli. Once colonies of these bacteria have increased, continued lactose exposure should maintain tolerability of lactose without further exposure to RP-G28.
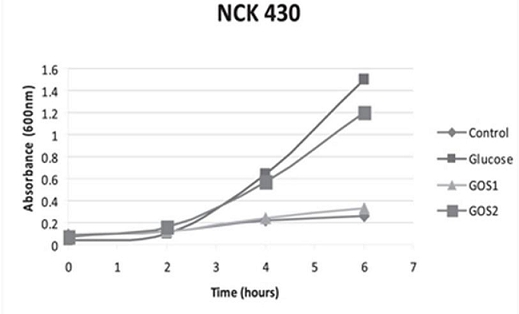
The significance of a higher purity GOS, namely RP-G28, was highlighted in a 2010 study by Klaenhammer. The in vitro study concluded that RP-G28 promoted growth of lactobacilli and bifidobacteria, but did not promote multiple strains of E. coli. In contrast, lower purity GOS stimulated both bifidobacteria as well as the strains of E. coli evaluated. (As seen below in Figure 1, NCK 430 (e. coli) grew in the presence of low purity GOS (GOS 2). Alternatively, the higher purity GOS (RP-G28/GOS 1) did not promote the growth of E. coli.).