SCYNEXIS
Overview
SCYNEXIS, Inc. (SCYX) is a biotechnology company committed to positively impacting the lives of patients suffering from difficult-to-treat and often life-threatening infections by delivering innovative anti-infective therapies. SCYNEXIS is developing its lead product candidate, SCY-078, as the first representative of a novel oral and intravenous (IV) triterpenoid antifungal family in clinical development for the treatment of several serious fungal infections, including invasive candidiasis, invasive aspergillosis, refractory invasive fungal infections and vulvovaginal candidiasis (VVC). SCY-078 is a structurally distinct glucan synthase inhibitor that has been shown to be effective in vitro and in vivo against a broad range of human fungi pathogens such as Candida and Aspergillus species, including multidrug-resistant strains, as well as Pneumocystis species. Candida and Aspergillus species are the fungi responsible for approximately 85% of all invasive fungal infections in the United States (U.S.) and Europe. To date, SCYNEXIS has characterized the pharmacokinetics and safety profile of oral and IV formulations of SCY-078 in multiple Phase 1 studies. In a Phase 2 study, evaluating oral SCY-078 as a step-down therapy in patients with invasive candidiasis, the company confirmed that oral SCY-078 achieved the intended plasma exposure for efficacy and was well-tolerated. In another Phase 2 proof-of-concept study, evaluating oral SCY-078 in patients with VVC, the company observed numerically higher clinical cure rates at test-of-cure visit and fewer recurrences of infection at the four-month follow-up when compared to oral fluconazole, the standard of care (SoC). The company applied to the U.S. Food and Drug Administration (FDA) for, and received, the designation of the oral tablet and IV formulations of SCY-078 for invasive candidiasis and invasive aspergillosis as Qualified Infectious Disease Product, or QIDP, under the Generating Antibiotic Incentives Now Act, or GAIN Act. The company also applied to the FDA for, and were granted, Fast Track designation for SCY-078 for these indications.1
Platform of Indications
The company continue to accelerate and expand its clinical programs, leveraging the versatility of the SCY-078 platform, including the potential for oral SCY-078 to be a suitable treatment for indications with significant unmet medical needs and considerable commercial opportunity. The following table summarizes the indications SCYNEXIS is currently seeking, anticipated NDA submission timing and estimated peak sales in the United States:
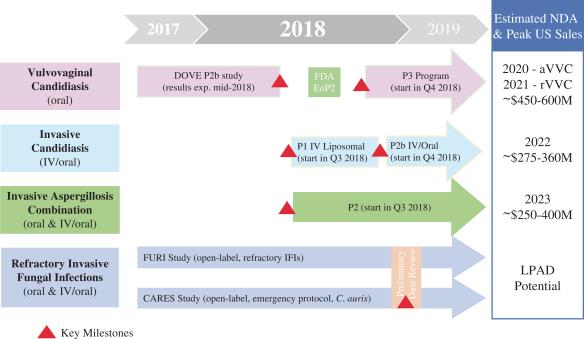
VVC—The company's most advanced stage of clinical development, targeting both acute and recurrent infections.
SCYNEXIS is currently enrolling patients in a Phase 2b dose-finding study of oral SCY-078 for the treatment of VVC (DOVE study). The DOVE study is a randomized, multicenter, double-blind, active-controlled, dose-finding study designed to evaluate the safety and efficacy of oral SCY-078 versus oral fluconazole in adult female patients. Approximately 180 patients with moderate to severe acute VVC are being randomized to one of five different regimens of oral SCY-078 or oral fluconazole, the current standard of care (SoC). Efficacy will be measured by the percentage of patients with clinical cure (complete resolution of signs and symptoms) at the test-of-cure visit at day 10 (primary endpoint) and at a follow-up visit on day 25. Mycological eradication (negative fungal culture) will also be evaluated at the same time points. Robust enrollment in the study has been maintained and the company expect to report top-line results for this study in mid-2018. If successful, following completion of the DOVE study and following an End-of-Phase 2 meeting with the FDA, the company plan to study the dose regimen selected from this study in a subsequent Phase 3 program, potentially initiating in the fourth quarter of 2018 with the objective of filing the New Drug Application (NDA) for acute VVC in 2020.
Invasive Candidiasis—Path forward established for IV program of SCY-078, with clinical trials to initiate in the third quarter of 2018 with an improved IV formulation.
As previously disclosed in March 2017, the FDA required it to hold the initiation of any new clinical studies with a cyclodextrin-based IV formulation of SCY-078 following the review of three mild-to-moderate inflammation-related thrombotic events observed in healthy volunteers receiving the highest dose level of a cyclodextrin-based IV formulation of SCY-078 in a Phase 1 study. Based on subsequent interactions with the FDA, the company completed a broad range of pre-clinical activities designed to identify whether the underlying cause of the thrombotic events was related to the active ingredient, SCY-078, or the administration regimen for the cyclodextrin-based IV formulation of SCY-078. Several pre-clinical studies showed that SCY-078 does not affect blood coagulation by itself, providing supporting evidence that the thrombotic events associated with the administration of the cyclodextrin-based IV formulation were triggered by vascular endothelium inflammation at the site of infusion where the concentration of the cyclodextrin-based formulation of SCY-078 was greatest.
In parallel, the company continued its pursuit of alternative IV formulations and accelerated the development of a new formulation based on liposomal technology that has been successfully used to improve systemic tolerability of other commercially available IV formulations, including IV antifungals. In comparing the cyclodextrin-based IV formulation head-to-head against the liposomal IV formulation of SCY-078 in pre-clinical evaluations, the liposomal formulation showed a superior profile for infusion-related and vascular inflammation tolerability. Based on these initial pre-clinical studies, the company believe that the liposomal IV formulation may offer significant clinical benefits over the cyclodextrin-based IV formulation and, therefore decided to focus its efforts on the advancement of the liposomal IV formulation of SCY-078. This decision was discussed with the FDA and a path forward was established. IND-enabling pre-clinical studies with the liposomal formulation are ongoing, and the company anticipate initiating a Phase 1 study in healthy volunteers with the liposomal IV formulation of SCY-078 in the third quarter of 2018. If successful, following completion of the Phase 1 study and pending FDA’s review, the company plan to initiate a Phase 2b IV-Oral step-down study of SCY-078 in invasive candidiasis patients with the liposomal IV and oral formulations of SCY-078 in the fourth quarter of 2018.
Invasive Aspergillosis—SCY-078 in combination with standard of care may represent a significant opportunity to improve outcomes for this high-mortality infection.
Based on promising pre-clinical data with combination use of SCY-078 with SoC vs. Aspergillus spp., the company plan to initiate a Phase 2 study of oral SCY-078 in patients with invasive aspergillosis in the third quarter of 2018. This initial study is planned as a randomized, double-blind trial with the objective of assessing the safety and efficacy of oral SCY-078 in combination with a mold active azole therapy, the SoC for this indication, compared to SoC alone. The company believe that SCY-078’s broad activity against Aspergillus spp., including azole-resistant strains, along with its minimal drug-drug interactions, high tissue penetration into the lungs and oral formulation allowing for long-term administration, may make it an ideal candidate for use as combination therapy to provide improved outcomes vs. SoC.
Refractory Invasive Fungal Infections—Potential for streamlined development pathway.
SCYNEXIS is currently enrolling patients in the FURI study, a global, open-label study in which oral SCY-078 is being administered to patients with invasive fungal infections that are refractory to, or that are intolerant of, standard therapy (azoles, echinocandins and/or polyenes). Twenty-four locations in the U.S. and Europe are now active in this study and enrollment is progressing. The company also initiated the CARES study, a global, open-label study of oral SCY-078 for the treatment of Candida auris infections. Candida auris has been classified by the Centers for Disease Control and Prevention (CDC) as a serious public health threat, as it is multidrug-resistant, has resulted in high mortality rates (up to 60%) and can be spread from patients (and surfaces) to patients, resulting in hospital outbreaks. The CARES study is intended to provide rapid access to oral SCY-078 for patients suffering from this life-threatening infection.
The open-label designs of the FURI and CARES studies allow for evaluation of the data on an interim basis to further inform subsequent regulatory steps of the development program. The company believe that compelling data from the FURI and/or CARES studies could allow SCY-078 to become eligible for the regulatory Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) potentially resulting in an initial NDA based on streamlined development. The company plan to continue to advance enrollment in the FURI and CARES studies, both in the U.S. and globally, with preliminary data review planned for the fourth quarter of 2018.
Key milestones
The company believe the company will achieve the following key milestones in 2018:
- to complete enrollment and announce top-line study results of the DOVE Phase 2b study of oral SCY-078 as a treatment for VVC in mid-2018 and initiate the Phase 3 VVC program in the fourth quarter of 2018 following an end-of-Phase 2 meeting with the FDA;
- to initiate the Phase 1 clinical trial to evaluate the safety and tolerability of the liposomal IV formulation of SCY-078 in healthy volunteers in the third quarter of 2018. If successful, following completion of the Phase 1 study, initiate a Phase 2b clinical trial designed to evaluate IV/oral SCY-078 for the treatment of invasive candidiasis in the fourth quarter of 2018;
- to initiate a Phase 2 study of oral SCY-078 in combination with current standard of care (azole) as a treatment for invasive aspergillosis in the third quarter of 2018; and
- to continue to advance enrollment in both the FURI and CARES studies, both in the U.S. and globally, with preliminary data review planned for the fourth quarter of 2018.
Strategy
Key elements of its strategy include:
- to further develop SCY-078 and obtain regulatory approval in major commercial markets for its key initial indications: VVC (acute and recurrent), invasive candidiasis and invasive aspergillosis;
- to commercialize SCY-078 for selected indications in the U.S. through a dedicated commercial team, including field force;
- to contract with commercial partners to develop and commercialize SCY-078 outside of the U.S.;
- to assess external opportunities to expand its clinical pipeline; and
- o leverage its strong scientific team to pursue the development of other internal proprietary compounds.
Market Opportunity
Acute and Recurrent VVC
VVC, commonly known as a “vaginal yeast infection,” is the second most common cause of vaginitis and it is usually caused by Candida spp. It affects approximately 70%-75% of women at least once in their lifetime, with 40-50% of these women experiencing more than one episode. VVC episodes include the following:
- Uncomplicated cases. These are sporadic mild-to-moderate infections typically caused by C. albicans spp. in a normal host. They represent the majority of the VVC episodes; and
- Complicated cases. These represent the remaining episodes and include: severe infections, recurrent cases, infections caused by non-albicans Candida spp., and/or observed in an abnormal host.
VVC can be associated with substantial morbidity, including significant genital discomfort, reduced sexual pleasure, psychological distress and loss of productivity. The global diagnosis and treatment, along with lost productivity, is estimated to cost $1.0 billion per year in the U.S.
Current treatments for acute VVC include OTC topical azole antifungals (clotrimazole, miconazole, and others) and the use of the prescription oral azole antifungal, fluconazole. Fluconazole is the only orally-administered antifungal currently approved for acute VVC in the U.S., with a therapeutic cure rate of 55% as reported in its label. Uncomplicated acute VVC cases are often effectively treated with topical agents and/or with one to three doses of oral fluconazole. However, management of VVC during pregnancy, moderate-to-severe VVC, recurrent VVC and VVC caused by fluconazole-resistant Candida spp., are not fully addressed by oral fluconazole. In addition, there are no oral alternatives for VVC patients who do not respond to or tolerate fluconazole, and there are no FDA-approved products for the treatment of recurrent VVC. The company believe that SCY-078, if approved for the treatment of acute and recurrent VVC, may provide a significant benefit for patients not satisfied with existing therapies.
Invasive Candidiasis
Invasive Candidiasis is a serious fungal infection caused by various species of the Candida infection that occurs in immunocompromised patients. The current treatment algorithm for invasive candidiasis infections includes empiric treatment, confirmed treatment, and maintenance step-down treatment as defined below:
- Empiric Treatment. The rapid progression of disease and high mortality rates associated with documented invasive fungal infections often result in antifungal therapy being administered in the hospital in suspected (unconfirmed) cases;
- Confirmed Treatment. Once a Candida infection is confirmed (via blood culture or rapid diagnostic) treatment begins in the hospital setting, as it occurs most commonly in ICU and surgical patients, patients using a central venous catheter, and immunosuppressed patients; and
- Maintenance Step-down Treatment. Depending on the risk factors of patients, some of them may be allowed to continue treatment with oral step-down therapy in the outpatient setting. Treatment should continue for two weeks after signs and symptoms have resolved and Candida yeasts are no longer in the bloodstream.
Current treatment guidelines for invasive candidiasis in the U.S. and in Europe recommend the use of IV echinocandins (the only glucan synthase inhibitor currently commercially available) as first-line therapy for empiric and confirmed cases. The main limitation of the echinocandin class is that only IV administration is available, limiting the flexibility of stepping down to an oral therapy in the same treatment class. The only option currently available to step down to an oral therapy after initial IV echinocandin are the azoles (the only antifungal class orally bioavailable).
Despite existing antifungal agents, mortality in this high-risk patient population remains high at approximately 30-40%. In addition, the increasing rate of drug-resistant Candida strains has created a need for new treatments. The CDC has listed fluconazole-resistant Candida as a serious threat requiring prompt and sustained action and has also identified a rise in echinocandin resistance, especially among Candida glabrata. In June 2016, the CDC issued an extraordinary alert for healthcare facilities and providers to be on the lookout for patients with C. auris, a multidrug-resistant strain with high mortality (approximately 60%). The company believe that SCY-078, if approved for the treatment of invasive candidiasis, may provide an alternative to current IV echinocandin use in empiric and confirmed cases, and fulfill the significant current unmet needs in the oral maintenance setting.
Invasive Aspergillosis
Invasive Aspergillosis is a serious fungal infection caused by Aspergillus species. The infection is reported to be the leading infection-caused death in immunocompromised patients. Current treatment guidelines in the U.S. and in Europe recommend the use of azoles (itraconazole, voriconazole or isavuconazole) as the initial first-line therapy. However, patients face unsatisfactory clinical outcomes with mortality rates ranging from 30% to 80% (depending on the stage of infection and the host underlying disease) and long treatment durations. Additionally, current therapies often exhibit drug-drug interaction, and the recent emergence of A. fumigatus azole resistance is increasingly becoming of clinical concern worldwide.
Due to the significant rate of resistance in some countries (i.e., Netherlands ~10-20%), combination antifungal therapy as first-line treatment for patients suspected of invasive aspergillosis is recommended. The combination of voriconazole or isavuconazole with an IV echinocandin is recommended at least until results of resistance testing are obtained. A previous study, by Marr et al. in invasive aspergillosis patients demonstrated that the combination of an IV echinocandin and an IV/oral azole for two weeks followed by an oral azole alone for four additional weeks improved outcomes in certain patient subgroups. In this study, the combination regimen was given for only two weeks because of the limitations of using an IV echinocandin long-term in the outpatient setting. The company believe that SCY-078, if approved in combination with standard of care for the treatment of invasive aspergillosis, would allow patients to receive the required combination treatment for the full six to twelve weeks of therapy, possibly leading to better outcomes.
SCY-078 Target Product Profile
SCY-078, a triterpenoid analogue, represents a new chemical class which acts through the inhibition of the glucan synthase, an established target in antifungal therapeutics. SCY-078 is being developed as oral and IV formulations and has demonstrated potent activity against a large collection of medically relevant strains of Candida and Aspergillus species, including multi-drug resistant strains, as well as Pneumocystis species. Additionally, SCY-078 has shown in vitro and in vivo activity against multi-drug resistant organism such as Candida auris and synergistic/additive activity in combination with isavuconazole against Aspergillus strains. SCY-078 has unique attributes that define its potential to address significant unmet medical needs and market opportunities, including:
- broad activity against Candida, Aspergillus, and Pneumocystis strains;
- activity against azole and most echinocandin-resistant Candida strains, including multi-drug resistant strains;
- activity against azole-resistant Aspergillus strains;
- only glucan synthase inhibitor with both oral and IV formulations in clinical development, allowing for first-line treatment, oral step-down with the same agent and longer duration of treatment;
- distinct chemical structure from other glucan synthase inhibitors, providing a unique spectrum of activity and pharmacokinetic profile;
- fungicidal (i.e., killing the fungi) capabilities against Candida species compared to azoles, which are fungistatic (i.e., inhibiting the growth of fungi);
- high tissue penetration, allowing high concentrations in the organs commonly affected by fungal infections; and
- nhanced activity at acidic pH (normal vaginal pH is 3.8 to 4.5).
The company believe that SCY-078, if approved, has the potential to address significant gaps with commercially available therapies in the following indications:
- acute moderate-severe and recurrent vulvovaginal candidiasis;
- invasive candidiasis (including resistant infections); and
- invasive aspergillosis (including resistant infections).
In the future, the company may also consider other indications for SCY-078 for which longer oral antifungal regimens are typically needed and would benefit from the broad spectrum of activity, favorable safety profile and low potential for drug-drug interactions, including for the treatment of chronic fungal infections and for prophylaxis use.
Treatment of VVC. If SCY-078 is approved for the treatment of VVC, it could provide a first-line therapy for recurrent VVC, for which there is currently no approved treatment, and be the only oral, non-azole, fungicidal treatment for moderate and severe acute cases of VVC. The company believe that SCY-078’s broad spectrum activity (including fluconazole-resistant strains), its enhanced activity at acidic pH and its high penetration in the vaginal tissue, may allow SCY-078 to address the current unmet needs in this indication and improve the quality-of-life of these patients. Additionally, in contrast with fluconazole that is fungistatic against Candida spp., SCY-078 is fungicidal against most Candida isolates. The company believe that SCY-078’s “cidal” activity (i.e., killing the pathogen) may provide an advantage in preventing recurrences.
Treatment of invasive Candida infections. If SCY-078 is approved for the treatment of invasive Candida infections, the company believe it could complement or replace IV echinocandins as the drug of choice for these infections because of its broader spectrum of activity and its availability in both IV and oral forms. Having both formulations would allow physicians and their patients to start and stay on a single effective therapy for both inpatient and outpatient settings. Transitioning patients from hospital-based care to outpatient care is key to potentially reduce, or eliminate, expensive hospital stays and risks of hospital-acquired infections. Given the growing emergence of fluconazole-resistant Candida in hospital settings, SCY-078 could also be used as the step-down therapy from any IV echinocandin, replacing fluconazole, and providing the advantage of continuing the antifungal treatment with an oral glucan synthase inhibitor that has a broader spectrum of activity than fluconazole.
Treatment of invasive Aspergillus infections. The company believe that SCY-078's broad activity against Aspergillus spp., including azole-resistant strains, along with its minimal drug-drug interactions, high tissue penetration into the lungs and oral formulation allowing for long-term administration, may make it an ideal candidate for use as combination therapy. If the combination of SCY-078 and standard of care is approved for the treatment of invasive Aspergillus infections and provides a significant improvement in clinical outcomes, “SCY-078 combo” could replace the azole as the treatment of choice for this difficult-to-treat infection. The company recently reported data showing synergistic activity of SCY-078 in combination with an azole in both in vitro and in vivo models of invasive aspergillosis. A previous study, by Marr et al. in invasive aspergillosis demonstrated that the combination of an IV echinocandin and an azole for two weeks followed by an oral azole alone for four additional weeks improved outcomes in certain patient subgroups. In this study, the combination regimen of an IV echinocandin with an azole was given for only two weeks, because of the limitations of using an IV echinocandin long-term in the outpatient setting. A combination of SCY-078 and an azole would allow patients with invasive aspergillosis to receive this combination for the full six to twelve weeks of therapy, possibly leading to better outcomes.
Treatment of refractory invasive fungal infections. SCY-078 has been shown to be effective pre-clinically against Candida species resistant to azoles, including C. auris, C. albicans, C. glabrata and C. krusei. In addition, SCY-078 has been shown to be effective in vitro against the majority of echinocandin-resistant Candida strains tested. Candida auris has been classified by the Centers for Disease Control and Prevention (CDC) as a serious public health threat, as it is multidrug-resistant, has resulted in high mortality rates (up to 60%) and can be spread from patients (and surfaces) to patients, resulting in hospital outbreaks. The current refractory invasive fungal infections open-label studies (CARES and FURI) may provide SCY-078 the opportunity to become eligible for the regulatory Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) potentially resulting in an initial New Drug Application (NDA) based on streamlined development. If approved, the company believe SCY-078 has the potential to become the treatment of choice in this patient population.
Given its stage of development, SCYNEXIS has not yet established a commercial organization or distribution capabilities.
For the treatment of VVC, the company anticipate that prescribing physicians will mostly be obstetricians and gynecologists and likely a number of primary care physicians and, the company believe, it may require a specific sales and marketing force with a women's health focus. The company will assess its global commercial strategy for VVC in the future.
For the treatment of invasive fungal infections, the company expect that prescribing physicians for the treatment of invasive fungal infections will be located at major medical centers, where physicians specializing in critical care, infectious disease specialists, and physicians treating immune compromised or immuno-suppressed patients, such as oncologists and those performing solid organ transplants and stem cell transplants, are likely to be found. For these indications, the company intend to form its own focused hospital-based field force to target physicians in the U.S. Outside of the U.S., subject to obtaining necessary marketing approvals, the company will likely seek to commercialize SCY-078 through distribution or other collaboration arrangements.
Competition for SCY-078
The company's competitors include large pharmaceutical and biotechnology companies, and specialty pharmaceutical and generic drug companies. The three leading branded antifungal drugs representing one from each main class are as follows:
Azoles (2016 worldwide sales of $800.0 million). Noxafil® (posaconazole) marketed by Merck and Cresemba® (isavuconazole), recently approved in the U.S. and other global markets and marketed by Astellas in the U.S.;
Echinocandins (2016 worldwide sales of $1.0 billion). Cancidas® (caspofungin), a product that became generic in March 2017. Pfizer also markets the echinocandin Eraxis® (anidulafungin) and Astellas markets the echinocandin Mycamine® (micafungin); and
Polyenes (2016 worldwide sales of $500.0 million). AmBisome® (liposomal amphotericin B), a product sold by Gilead in Europe, by Astellas in the U.S. and by Dainippon-Sumitomo in Japan.
Pfizer, Merck, Astellas, and Gilead are all large pharmaceutical companies with significant experience and financial resources in the marketing and sale of specialty pharmaceuticals. Various other producers market and sell generic oral voriconazole, fluconazole and itraconazole.
Further, the company expect that product candidates currently in clinical development may represent significant competition, if approved. These include the triazole VT-1161 being developed by Viamet Pharmaceuticals, Inc. (assets recently acquired by NovaQuest Capital Management, LLC), the long-acting IV echinocandin CD101 being developed by Cidara Therapeutics, Inc., APX-001 developed by Amplyx Pharmaceuticals Inc., the polyene amphotericin B oral formulation MAT2203 developed by Matinas BioPharma Holdings Inc., F901318 developed by F2G Limited and VL2397 developed by Vical Incorporated. These companies may have greater resources than ours.
The company believe that SCY-078 has the ability to perform well in the future fungal infection market given the sparse competitive marketplace, the unmet medical need, and the high mortality rate of these infections. The key competitive factors affecting the success of SCY-078, if approved, are likely to be its efficacy, safety, convenience, price, use in outpatient settings, the level of generic competition and the availability of reimbursement from government and other third-party payors. If approved, the company believe that SCY-078’s unique features, including being a novel antifungal class, broad-spectrum of activity including resistant strains, IV and oral formulations, fungicidal activity versus Candida, high tissue penetration, and favorable safety profile, will differentiate it from competing products and allow premium pricing to generics and other competing products.
The company's commercial opportunity could be reduced or eliminated if its competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than products that the company may develop. The company's competitors also may obtain FDA, or other regulatory, approval for their products more rapidly than the company may obtain approval for ours. In addition, its ability to compete may be affected because in many cases insurers or other third-party payors seek to encourage the use of generic products. In the azole class, fluconazole, itraconazole, and oral voriconazole are generic. Caspofungin, the largest selling echinocandin, is now available on a generic basis. If approved, the company believe SCY-078 will be capable of delivering value supportive of premium pricing over competitive generic products.
SCY-078 Development
The company initially discovered and developed SCY-078 through a research collaboration with Merck Sharp & Dohme Corp., or Merck, a subsidiary of Merck & Co., Inc., and in May 2013 the company acquired worldwide rights to SCY-078 in the field of human health. The compound is derived, by chemical modification, from enfumafungin, a natural product, and shows antifungal activity against Candida and Aspergillus through inhibition of glucan synthesis, a similar mechanism of action to the echinocandin class. SCY-078 has shown fungicidal activity against clinically relevant Candida species and potent in vitro activity against strains of Candida that are resistant to azoles and echinocandins and azole-resistant Aspergillus species. SCYNEXIS has reported potent antifungal in vitro activity of SCY-078 against the multidrug resistant pathogen Candida auris, which has been classified by the CDC as an emerging serious global health threat. SCY-078 is the first representative of new class of antifungal agents, triterpenoid analogue (a novel and structurally distinct glucan synthase inhibitor), that retains antifungal activity against the majority of echinocandin-resistant strains, suggesting that SCY-078 acts on the fungal cell wall in a manner distinct from the echinocandins.
SCYNEXIS is developing both IV and oral formulations of SCY-078. Patients with invasive fungal infections are typically prescribed IV treatment in hospitals and then are switched, or “stepped down,” to oral formulations to complete their antifungal treatment after they have shown sufficient improvement. The duration of the entire antifungal regimen (IV and oral) varies depending on the response to the antifungal treatment and the type of infection. Per current guidelines, invasive candidiasis patients are treated for at least two weeks after negative cultures are obtained and invasive aspergillosis patients are typically treated for six to 12 weeks. The availability of SCY-078 in both oral and IV formulations would allow for maximum flexibility in the administration of the same agent during the entire antifungal regimen. The IV formulation would allow initiation of treatment in critically ill patients for whom IV therapy is preferred. The oral formulation would allow step-down from the initial IV antifungal agent (either SCY-078 or echinocandins) to complete the antifungal regimen, as well as initiation of therapy in outpatient settings for those conditions that do not require hospitalization, such as VVC.
In animal models of invasive fungal infections used to test other drugs that have proven to be effective in humans, SCY-078 was shown to be highly active against Candida spp. These studies determined the drug concentrations in blood required to achieve efficacy. These correlations of drug exposure to drug activity, or PK/PD, have been used to identify the predicted human exposure of SCY-078 believed to be required to achieve efficacy (i.e., target exposure). Phase 1 and Phase 2 studies of SCY-078 indicate that the target exposure is achievable in humans at doses and regimens expected to be safe and adequately tolerated.
To date, more than 400 subjects and patients have received SCY-078 either orally, by IV, or by IV followed by oral. The most commonly reported adverse events after oral administration have been gastrointestinal events (i.e., nausea, diarrhea, vomiting). The gastrointestinal (GI) events reported have typically been transient (i.e., short duration), mild or moderate and not leading to discontinuation. The most commonly reported adverse events after IV administration of SCY-078 have been local reactions at the site of infusion. During its Phase 1 IV program in healthy volunteers, the company observed three mild-to-moderate thrombotic events in healthy volunteers receiving the cyclodextrin-based IV formulation of SCY-078 at the highest doses and highest concentrations in a Phase 1 study. Based on these events, the FDA required it to hold the initiation of any new clinical studies with the IV formulation of SCY-078. The company completed a broad range of pre-clinical activities designed to identify the underlying cause of the thrombotic events and to evaluate the optimal administration regimen for IV formulations of SCY-078. Several pre-clinical studies showed that SCY-078 does not affect blood coagulation by itself, providing supporting evidence that the thrombotic events were triggered by vascular endothelium inflammation at the site of infusion. The company identified a new formulation based on liposomal technology that its preclinical evaluations indicate has a superior profile for infusion-related and vascular inflammation tolerability when compared with the cyclodextrin-based IV formulation. Subsequent development activities for the IV formulation will be carried out with the new liposomal based formulation.
Serious Adverse Events (SAEs) are common when conducting clinical trials in a seriously ill population such as patients experiencing invasive candidiasis. Several SAEs have been reported in its clinical trials but only four of the events have been deemed by the investigator to be potentially related to SCY-078, although other contributing factors could not be ruled out. These four SAEs include: one event of elevation of liver function tests in a subject who received a single dose of oral SCY-078 (resolved) and three events secondary to thrombi formation at site of IV infusion (resolved) using the cyclodextrin-based IV formulation of SCY-078.
SCY-078 is protected by an issued composition of matter patent in the United States, which expires in 2030. The composition of matter patent has been granted in 63 countries and is pending in 19 other countries. Additional patent applications related to SCY-078 salts and polymorphs, and its use as an antifungal agent, have been filed and are currently pending. If granted, the new patent families will extend the patent protection of SCY-078 salts, including the citrate salt currently under development, up to 2036.
Preclinical Characterization of SCY-078
SCY-078 has broad antifungal activity based on a proven mechanism of action
SCY-078 is a potent inhibitor of the synthesis of the polymer beta (1,3)-D-glucan, an essential component of the fungal cell walls of Candida and Aspergillus species. Glucan synthesis inhibition is a clinically proven antifungal mechanism of action, as demonstrated by the echinocandin class of antifungal agents. The activity of SCY-078 observed against the majority of echinocandin-resistant strains suggests that SCY-078 acts in a manner distinct from the echinocandins. SCY-078 has been shown to have potent activity in vitro against clinically relevant Candida and Aspergillus species, including isolates that are resistant to currently available antifungal therapies. Azole-resistance among Candida and Aspergillus species is a global concern, particularly considering that azoles are the only antifungal class used to treat these life-threatening conditions that can be administered orally. SCY-078 retains its antifungal potency against fungal strains that are resistant to azoles. Echinocandin resistance is increasing in prevalence, particularly among azole-resistant species such as Candida glabrata. SCY-078 retained in vitro activity against a majority of echinocandin-resistant Candida glabrata strains SCYNEXIS has tested. Multidrug resistance has been reported in several strains of Candida; particularly concerning is the emergence of C.auris that exhibits high rates of resistance to two or more antifungals. Thus, SCY-078 may offer a therapeutic option against multidrug resistant strains such as those that have emerged in C. glabrata and C. auris. In addition, SCY-078 has shown to have activity against Pneumocystis spp.
Nonclinical toxicology is supportive of continued development
The preclinical safety of SCY-078 has been evaluated in multiple studies in rats, dogs, rabbits, and non-human primates. The SCY-078 toxicology program was expanded to include three-month oral dose studies in two species (rats and dogs). Consistent with findings from prior non-clinical toxicology studies of shorter durations, the company believe that these longer-term toxicity studies confirmed the favorable safety profile of oral SCY-078. Chronic (six-month rat, nine-month dog) oral dose studies are currently ongoing. These studies will allow flexible treatment regimens of SCY-078 beyond three months in its next stages of clinical development, which is particularly relevant for patients with invasive aspergillosis or refractory fungal infections.
Manufacturing and Supply of SCY-078
SCYNEXIS has agreements with external vendors that are capable of supplying kilogram quantities of drug substance and of producing drug product to support ongoing and planned clinical trials. However, the company do not own or operate and do not intend to own or operate facilities for manufacturing, storage and distribution, or testing of drug substance or drug product. SCYNEXIS has relied on third-party contract manufacturers for synthesis of its clinical compounds and manufacture of drug product. The company expect to continue to rely on either existing or alternative third-party manufacturers to supply SCY-078 for ongoing and planned clinical trials and for commercial production.
SCY-078 is a semi-synthetic compound. Thus, the manufacturing process for SCY-078 involves fermentation and synthetic chemical steps. The synthetic process does not require any specialized equipment and uses readily sourced intermediates. At commercial launch, the company expect cost of goods for SCY-078 to be similar to that of other small molecule drugs. SCYNEXIS has negotiated agreements with suppliers to produce both drug product and drug substance for its current needs. In the future, the company plan to validate the process with selected vendors and secondary suppliers to establish a secure supply chain that could enable commercialization.
The company estimate its supplies on hand for both oral and IV formulations of SCY-078 are sufficient to supply its ongoing and planned clinical trials. Manufacture of additional supplies of SCY-078 drug substance is planned to support any further optimization of either of the formulations, if needed. Additional batches of both oral and IV SCY-078 drug product will be manufactured as needed to support the subsequent stages of its clinical development plan.
A drug manufacturing program subject to extensive governmental regulations requires robust quality assurance systems and experienced personnel with the relevant technical and regulatory expertise as well as strong project management skills. The company believe SCYNEXIS has a team that is capable of managing these activities. The third-party vendors that currently manufacture clinical supplies to support its ongoing clinical studies have the necessary capabilities and are in compliance with cGMP appropriate for the current stage of development.
The third-party vendors the company will select to support its manufacturing and supply program both for future late-stage development and commercial readiness activities will have the required capabilities with respect to facilities, equipment and technical expertise, quality systems that meet global regulatory and compliance requirements, satisfactory regulatory inspection history from relevant health authorities and proven track records in supplying drug substance and drug product for late-stage clinical and commercial use.
Research and Development
A significant portion of its operating expenses is related to research and development and the company intend to maintain its strong commitment to research and development. In fiscal years 2017 and 2016, the company incurred $18.3 million and $20.1 million, respectively, on research and development expenses. See "Item 8. Financial Statements and Supplementary Data" of this Annual Report on Form 10-K for costs and expenses related to research and development, and other financial information for each of the fiscal years 2017 and 2016.
Collaborations and Licensing Agreements Associated with The company's Core Drug Development Operations
The company currently have a number of licensing and collaboration agreements associated with its core drug development operations, including the following:
Merck
The company initially discovered and developed SCY-078 through a research collaboration with Merck Sharp & Dohme Corp., or "Merck", a subsidiary of Merck & Co., Inc. In May 2013, Merck transferred to it all development and commercialization rights for SCY-078 (also known as MK-3118). This decision was made following a review and prioritization of Merck’s infectious disease portfolio. Under the terms of the agreement, the company received all human health rights to SCY-078, including all related technical documents, preclinical data, data from the seven Phase 1 trials conducted by Merck, and drug product and drug substance. The agreement continues until expiration of all royalty obligations. The agreement may be terminated if either party is in material breach and fails to remedy the breach after receiving written notice. In January 2014, Merck assigned the patents to it related to SCY-078 that it had exclusively licensed to it. Under the terms of the patent assignment, Merck no longer has responsibility to maintain the patents. Merck is eligible to receive milestones upon initiation of a Phase 3 clinical study, NDA filing and marketing approvals in each of the U.S., major European markets and Japan that could total up to $19 million. In addition, Merck will receive tiered royalties based on worldwide sales of SCY-078. The aggregate royalties are in the single digit percentages of net sales, and the company expect to pay royalties on net sales of SCY-078 to Merck for no more than ten years from first commercial launch, on a country-by-country basis.
In December 2014, the company entered into an amendment to the license agreement with Merck that defers the remittance of a milestone payment due to Merck, such that no amount will be due upon initiation of the first phase 2 clinical trial of a product containing the SCY-078 compound (the "Deferred Milestone"). The amendment also increased, in an amount equal to the Deferred Milestone, the milestone payment that will be due upon initiation of the first Phase 3 clinical trial of a product containing the SCY-078 compound. In December 2016 and January 2018, the company entered into second and third amendments to the license agreement with Merck which clarified what would constitute the initiation of a Phase 3 clinical trial for the purpose of a milestone payment. Except as described above, all other terms and provisions of the license agreement remain in full force and effect.
R-Pharm
In August 2013, the company entered into an agreement with R-Pharm, CJSC, or "R-Pharm", a leading supplier of hospital drugs in Russia, granting them exclusive rights to develop and commercialize SCY-078 in the field of human health in Russia, Turkey, and certain Balkan, Central Asian, Middle Eastern and North African countries. The company retained the right to commercialize SCY-078 in the Americas, Europe, and Asia. The company received an upfront payment of $1.5 million and are entitled to receive up to $18 million in payments if certain development and sales based milestones are achieved. SCYNEXIS is also entitled to single digit percent royalty payments for products that do not fall under the patents and a royalty percentage in the teens for products that do fall under the patents. This agreement expires upon R-Pharm’s last royalty payment, which is the later of 12 years from the first registration of the product in the countries where R-Pharm’s license rights exist under this agreement, or the last to expire of the patents in such countries. Either party may terminate this agreement if the other party breaches and fails to remedy the breach after receiving notice from the non-breaching party. SCYNEXIS has the ability to terminate this agreement if the company determine that R-Pharm fails to make reasonable progress in the development and commercialization of SCY-078. If the company give R-Pharm notice of failure to make reasonable progress, R-Pharm will have the opportunity to correct the deficiencies.
The original agreement also included terms whereby R-Pharm would reimburse it for certain research and development costs associated with Phase 2 and Phase 3 clinical trials of oral SCY-078 and the development of an IV formulation of SCY-078. However, these cost reimbursement terms required that the clinical trials and the IV formulation development follow a global development plan that was agreed upon by both parties in August 2013. Subsequent to August 2013, modifications were made to the global development plan that caused the clinical trial cost reimbursement terms in the original agreement to no longer be enforceable. Further, the IV formulation development cost reimbursement terms in the original agreement did not specify which IV formulation and development costs were reimbursable by R-Pharm. In November 2014, the company entered into a supplemental arrangement with R-Pharm, whereby R-Pharm was informed of the modified IV formulation development plan and R-Pharm agreed to reimburse it for specifically identified IV formulation development and manufacturing costs incurred by it. The specifically identified costs were defined as all costs incurred by it under a separate arrangement SCYNEXIS has with a third-party service provider, whereby the third-party service provider is performing certain IV formulation and development services for it. The company estimate that total reimbursable costs pursuant to the original agreement and supplemental arrangement with R-Pharm will be approximately $1.3 to $1.9 million.
Intellectual Property
The company strive to protect the proprietary technology that the company believe is important to its business, including seeking and maintaining patents intended to cover its product candidates and compositions, and their methods of use and other inventions that are commercially important to the development of its business. The company also rely on trade secrets to protect aspects of its business that are not amenable to, or that the company do not consider appropriate for, patent protection.
As of March 1, 2018, SCYNEXIS is the owner of 8 issued U.S. patents and 118 issued non-U.S. patents with claims to novel compounds, compositions containing them, processes for their preparation, and their uses as pharmaceutical agents, with terms expiring between 2019 and 2036. Of these patents, one U.S. patent relates to SCY-078. SCYNEXIS is actively pursuing four U.S. patent applications and 22 non-U.S. patent applications in at least 19 jurisdictions.
The company's success will depend significantly on its ability to obtain and maintain patents and other proprietary protection for commercially important technology, inventions and know-how related to its business, defend and enforce its patents, maintain its licenses to use intellectual property owned by third parties, preserve the confidentiality of its trade secrets and operate without infringing the valid and enforceable patents and other proprietary rights of third parties. The company also rely on know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen, and maintain its proprietary position in the field of antifungal agents.
The company believe that SCYNEXIS has a strong intellectual property position and substantial know-how relating to the development and commercialization of SCY-078, including patents or patent applications covering inventions that SCYNEXIS has co-invented with Merck. The company cannot be sure that patents will be granted with respect to any of its pending patent applications or with respect to any patent applications filed by it in the future, nor can the company be sure that any of its existing patents or any patents that may be granted to it in the future will be commercially useful in protecting its technology.
The company's objective is to continue to expand its intellectual property estate by filing patent applications directed to SCY-078 or derivatives thereof. The company intend to pursue, maintain, and defend patent rights, whether developed internally or licensed from third parties, and to protect the technology, inventions, and improvements that are commercially important to the development of its business.
SCY-078
The patent portfolio for SCY-078 is directed to cover compositions of matter, formulation, methods of use and precursors or intermediaries in its preparation. This patent portfolio includes an issued U.S. patent and corresponding foreign national and regional counterpart patents and patent applications. The patents and patent applications relating to SCY-078 include patents and patent applications that were initially assigned to it and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc. Merck Sharp & Dohme Corp. subsequently assigned to it all of its rights in these patents and patent applications relating to SCY-078. The issued composition of matter patent (U.S. Patent No. 8,188,085), if the appropriate maintenance, renewal, annuity, and other governmental fees are paid, is expected to expire in 2030. Based on its current development plan, the company believe that an additional term of up to five years for the SCY-078 U.S. patent may result from the patent term extension provision of the Drug Price Competition and Patent Term Restoration Act of 1984 (the Hatch-Waxman Act). The company expect that the patent applications in this portfolio, if issued, and if appropriate maintenance, renewal, annuity, and other governmental fees are paid, would expire between 2030 and 2036, including any additional term from patent term adjustment or patent term extension. The patent term calculation method and the provisions under the Hatch-Waxman Act are described in the “Patent Term” section below. SCYNEXIS is not currently aware of any third-party patents (other than patents SCYNEXIS has licensed) encompassing SCY-078.
The terms of issued SCY-078 composition of matter patents in other jurisdictions (Algeria, Armenia, Australia, Azerbaijan, Belarus, Belize, Brunei, Canada, China, Colombia, El Salvador, EPO (Austria, Belgium, Croatia, Czech Republic, Denmark, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Luxembourg, Macedonia, Netherlands, Poland, Portugal, Serbia, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, United Kingdom), Hong Kong, Honduras, Indonesia, Israel, Japan, Kyrgyzstan, Korea, Kazakhstan, Lebanon, Morocco, Moldova, Mexico, Mexico, Malaysia, Nicaragua, New Zealand, Peru, Philippines, Russia, Singapore, South Africa, Tajikistan, Turkmenistan, Tunisia, Taiwan and Ukraine), if the appropriate maintenance, renewal, annuity, and other government fees are paid, are expected to expire in 2029. These patents and patent applications (if applicable), depending on the national laws, may benefit from extension of patent term in individual countries. In some European countries, for example, a supplementary protection certificate, if obtained, provides a maximum of five years of market exclusivity. The duration of the supplementary protection certificate may be extended to five and a half years when the supplementary protection certificate relates to a human medicinal product for which data from clinical trials conducted in accordance with an agreed Pediatric Investigation Plan, or PIP, have been submitted. Likewise, in Japan, the term of a patent may be extended by a maximum of five years in certain circumstances.
Patent Term
The term of individual patents and patent applications will depend upon the legal term of the patents in the countries in which they are obtained. Generally, the patent term is 20 years from the date of filing of the patent application (or earliest filed parent application, if applicable).
Under the Hatch-Waxman Act, the term of a patent that claims an FDA-approved drug may also be eligible for patent term extension, or PTE. Eligibility for a PTE is based, in part, on whether the FDA approval of the drug represents the first permitted commercial marketing or use of the drug. Drugs that are considered to be new chemical entities under FDA’s regulations are generally eligible for PTE.
TE permits patent term restoration of a U.S. patent as partial compensation for patent term lost during the FDA regulatory review process, which includes both the testing period while the drug is being investigated under an IND and the approval period while FDA is reviewing a marketing application. The length of the patent term extension is half the testing period plus all of the approval period, with certain limitations. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent; however, a patent term extension cannot in any event extend the remaining term of a patent beyond a total of 14 years from the date of product approval; only one patent that claims an approved drug may be extended; and the applicable approval must be the first approval of the product under the provision of law authorizing the approval. During the extension period, the patent holder’s rights under the patent are generally limited to approved uses of the product. Similar provisions may be available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug. When possible, depending upon the length of clinical trials and other factors involved in the filing of an NDA, the company expect to apply for patent term extensions for patents covering SCY-078 and its use in treating various diseases. As a specific example, if SCYNEXIS is awarded the maximum length of PTE, its U.S. composition of matter patent relating to SCY-078 would have an expected expiration date of the earlier of 14 years from product approval or August 28, 2035. However, depending on any changes in its clinical path and the date of FDA approval, the PTE may not be granted, or may be less than the maximum.
Proprietary rights and processes
The company may rely, in some circumstances, on proprietary technology and processes (including trade secrets) to protect its technology. However, these can be difficult to protect. The company seek to protect its proprietary technology and processes, in part, by entering into confidentiality agreements with its employees, consultants, scientific advisors, contractors, and collaborators. The company also seek to preserve the integrity and confidentiality of its proprietary technology and processes by maintaining physical security of its premises and physical and electronic security of its information technology systems. While SCYNEXIS has confidence in these individuals, organizations and systems, agreements or security measures may be breached, and the company may not have adequate remedies for any breach. In addition, its proprietary technology and processes may otherwise become known or be independently discovered by competitors. To the extent that its employees, consultants, scientific advisors, contractors, or collaborators use intellectual property owned by others in their work for it, disputes may arise as to the rights in related or resulting know-how and inventions. For this and more comprehensive risks related to its proprietary technology and processes, please see the section on “Risk Factors-Risks Relating to The company's Intellectual Property.”
Employees
As of March 1, 2018, the company had 19 employees, all of whom were employed on a full-time basis. The company's employees are engaged in administration, accounting and finance, research, clinical development, manufacturing, and business development functions. The company believe its relations with its employees are good.




