Sangamo BioSciences
OVERVIEW
Sangamo BioSciences (SGMO) is a clinical stage biotechnology company focused on translating ground-breaking science into genomic therapies that transform patients’ lives using its industry-leading platform technologies in genome editing, gene therapy, gene regulation and cell therapy.
Sangamo BioSciences is a leader in the research and development of zinc finger proteins, or ZFPs, a naturally occurring class of proteins found in humans. Sangamo BioSciences has used its knowledge and expertise to develop a proprietary technology platform in both genome editing and gene regulation. ZFPs can be engineered to make zinc finger nucleases, or ZFNs, proteins that can be used to specifically modify DNA sequences by adding or knocking out specific genes, or genome editing, and ZFP transcription factors or ZFP TFs, proteins that can be used to increase or decrease gene expression, or gene regulation. In the process of developing this platform, Sangamo BioSciences has accrued significant scientific, manufacturing and regulatory capabilities and know-how that are generally applicable in the broader field of gene therapy and have capitalized this knowledge into a conventional gene therapy platform based on adeno-associated viral vector, or AAV, cDNA gene transfer.1
The company's strategy is to maximize the value and therapeutic use of its technology platforms. In certain therapeutic areas the company intend to capture the value of its proprietary genome editing and gene therapy products by forward integrating into manufacturing, development and commercial operations. In other therapeutic areas the company intend to partner with biopharmaceutical companies to develop products.
Sangamo BioSciences is focused on the development of human therapeutics for diverse diseases with well-characterized genetic causes. Sangamo BioSciences has several proprietary clinical and preclinical product candidates in development and have strategically partnered certain programs with biopharmaceutical companies to obtain funding for its own programs and to expedite clinical and commercial development.
Sangamo BioSciences has an ongoing Phase 1/2 clinical trial evaluating SB-525, a gene therapy for the treatment of hemophilia A, a bleeding disorder. Sangamo BioSciences has ongoing Phase 1/2 clinical trials evaluating three product candidates using its proprietary in vivo genome editing approach: SB-FIX, for the treatment of hemophilia B, a bleeding disorder, SB-318, for the treatment of Mucopolysaccharidosis Type I, or MPS I, and SB-913 for the treatment of Mucopolysaccharidosis Type II, or MPS II. MPS I and MPS II are rare lysosomal storage disorders, or LSDs. Sangamo BioSciences is also initiating a Phase 1/2 clinical trial evaluating ST-400, developed using its proprietary ZFN-mediated ex vivo cell therapy platform, for the treatment of beta-thalassemia, a blood disorder. In addition, Sangamo BioSciences has proprietary preclinical and discovery stage programs in other LSDs and monogenic diseases, including certain central nervous system disorders, cancer immunotherapy, immunology and infectious disease.
In addition, Sangamo BioSciences has proprietary preclinical programs in other monogenic diseases and LSDs. The company's preclinical discovery efforts include research into potential therapeutic applications of its technology for certain central nervous system disorders, autoimmune disorders, infectious disease, and others.
In February 2018, the company entered into a global collaboration and license agreement with Kite Pharma, Inc., or Kite, a wholly-owned subsidiary of Gilead Sciences, Inc., or Gilead, for the research, development and commercialization of potential engineered cell therapies for cancer. In this collaboration, the company will work together with Kite on a research program under which the company will design ZFNs and AAVs to disrupt and insert certain genes in T cells and natural killer, or NK, cells, including the insertion of genes that encode chimeric antigen receptors, or CARs, T-cell receptors, or TCRs and NK-cell receptors, or NKRs, directed to mutually agreed targets. Kite will be responsible for all clinical development and commercialization of any resulting products.
In December 2017, the company entered into a new research collaboration and license agreement with Pfizer Inc., or Pfizer, for the development and commercialization of potential gene therapy products that use ZFP TFs to treat amyotrophic lateral sclerosis, or ALS, and frontotemporal lobar degeneration, or FTLD, linked to mutations of the C9ORF72 gene. Under this agreement, Sangamo BioSciences is working with Pfizer on a research program to identify, characterize and preclinically develop ZFP TFs that satisfy pre-agreed criteria. Pfizer is responsible for subsequent development, manufacturing and commercialization of licensed products.
In May 2017, the company entered into a global collaboration and license agreement with Pfizer for the research, development and commercialization of SB-525, its gene therapy product candidate for hemophilia A, and closely related products. Under this agreement, Sangamo BioSciences is responsible for conducting the Phase 1/2 clinical trial and certain manufacturing activities for SB-525, while Pfizer is responsible for subsequent worldwide development, manufacturing, marketing and commercialization of SB-525. The company and Pfizer may also collaborate in the research and development of additional AAV-based gene therapy products for hemophilia A.
Sangamo BioSciences has also established a collaborative partnership with Bioverativ, Inc., or Bioverativ, to research, develop and commercialize therapeutic gene-edited cell therapy products in hemoglobinopathies, including beta-thalassemia and sickle cell disease, or SCD. The company expect to begin enrolling patients in a Phase 1/2 clinical study in the first half of 2018. Bioverativ is responsible for subsequent development, manufacturing and commercialization of licensed products.
Sangamo BioSciences has a substantial intellectual property position in the genome editing field including the design, selection, composition and use of engineered ZFPs to support its research and development activities. As of February 15, 2018, the company either owned outright or have exclusively licensed the commercial rights to over 860 patents issued in the United States and foreign jurisdictions, and over 610 patent applications pending worldwide. The company continue to license and file new patent applications that strengthen its core and accessory patent portfolio. The company believe that its intellectual property position is a critical element in its ability to research, develop and commercialize products and services based on genome editing, gene therapy, gene regulation and cell therapy.
In January 2017, the company changed its corporate name to “Sangamo Therapeutics, Inc.” to underscore its focus on clinical development of genomic therapies using its industry-leading platform technologies across genome editing, gene therapy, gene regulation and cell therapy.
INTRODUCTION TO GENOME EDITING, GENE THERAPY, CELL THERAPY AND GENE REGULATION
DNA, Genes, and Proteins
Deoxyribonucleic acid, or DNA, is present in all cells except mature red blood cells, and encodes the inherited characteristics of all living organisms. A cell’s DNA is organized in chromosomes as thousands of individual units called genes. Genes encode proteins, which are assembled through the process of transcription—whereby DNA is transcribed into ribonucleic acid, or RNA,—and, subsequently, translation—whereby RNA is translated into protein (Figure 1). Proteins are involved in virtually all cell functions. DNA, RNA and
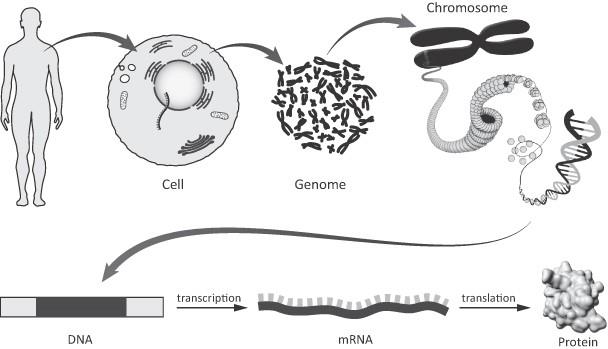
Schematic of the relationship between the human genome, DNA, RNA and protein
he human body is composed of specialized cells that perform different functions and are thus organized into tissues and organs. All somatic cells in an individual’s body contain the same set of genes. However, only a fraction of these genes are turned on, or expressed, in an individual human cell at any given time. Genes are regulated (i.e. turned on or turned off) by DNA-binding proteins called transcription factors in response to a wide variety of stimuli and developmental signals. Distinct sets of genes are expressed in different cell types. It is this pattern of gene expression that determines the structure, biological function and health of all cells, tissues and organisms. The aberrant expression of certain genes can lead to disease. Similarly, a mistake, or mutation in the DNA sequence of a gene, can result in corresponding error in the protein encoded by the gene, which may have serious consequences for the cell and its function. A number of disorders have been identified as caused by the inheritance of a single defective gene. These so-called monogenic diseases include hemophilia A and hemophilia B, LSDs such as MPS I and MPS II, beta-thalassemia, SCD, Huntington’s disease and many others.
ZFPs are Naturally Occurring Transcription Factors in Humans
A transcription factor recognizes and binds to a specific DNA sequence within or near a particular gene and causes expression of that gene to be “turned on” (activated) or “turned off” (repressed). ZFPs are the most common class of naturally occurring transcription factors in organisms from yeast to humans. In higher organisms, naturally occurring transcription factors typically comprise two domains: the first is a DNA-binding domain, (designated in Figure 2 as the “Recognition Domain”), which recognizes a target DNA sequence and thereby directs the transcription factor to the proper chromosomal location; the second is a functional domain that causes the target gene to be activated or repressed. To these naturally occurring transcription factors, Sangamo BioSciences has added functional domains which enable genome editing at the site determined by the ZFP DNA-binding domain.
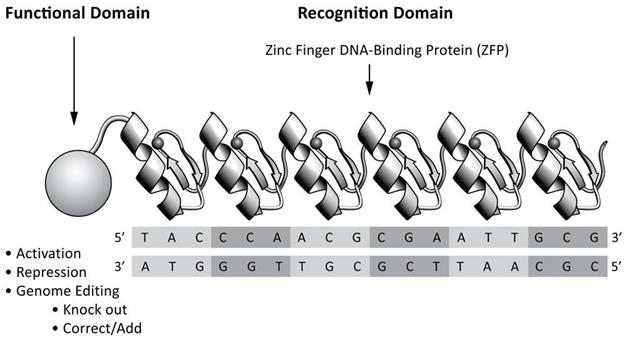
Schematic of the two-domain structure of a ZFP and its therapeutic functional domain
ZFNs can be designed for genome editing and ZFP TFs can be designed for gene regulation
Consistent with the modular structure of natural ZFPs, the company take a modular approach to the design of the proteins that the company engineer. The ZFP portion of its engineered proteins, the DNA-recognition domain, is typically composed of four to six zinc fingers. Each individual finger recognizes and binds to a three or four base pair sequence of DNA and multiple fingers can be linked together to recognize longer stretches of DNA, thereby improving specificity. By modifying the amino acid sequence of a ZFP, the company can engineer novel ZFPs capable of recognizing the DNA sequences of a chosen genomic target. The company use the engineered ZFP DNA-binding domain to link to a functional domain. The ZFP DNA-binding domain brings the functional domain to the target of interest. The company's ability to use its highly specific ZFP technology to precisely target a DNA sequence in a gene of interest provides it with a range of genome editing and gene regulation functions that can be applied in many different cell types.
The company's engineered ZFPs can be attached to a cleavage domain of a restriction endonuclease, an enzyme that cuts DNA, creating a ZFN. When a pair of ZFNs is bound to the DNA in the correct orientation and spacing, the DNA sequence is cut between the ZFP binding sites. DNA binding by both ZFNs is necessary for cleavage, and both nuclease of the restriction endonuclease must be present in the correct orientation to interact with each other, in order to mediate DNA cleavage. This break in the DNA triggers a natural process of DNA repair in the cell. The repair process can be harnessed to achieve one of several outcomes that may be therapeutically useful (Figure 3). If cells are simply treated with ZFNs alone, the repair process joins the two ends of the broken DNA together and frequently results in the loss or addition of a small amount of genetic material at the site of the break. This disrupts the original DNA sequence and can result in the expression of a truncated or non-functional protein from the targeted gene, effectively “knocking out” the gene function. ZFN-mediated genome editing can be used to disrupt genes that are involved in disease pathology. Sangamo BioSciences is using ZFN-mediated genome editing of the BCL11A erythroid enhancer in hematopoietic stem progenitor cells, or HSPCs, which is designed to be a single long-lasting treatment for beta-thalassemia (ST-400) and SCD (BIVV-003).
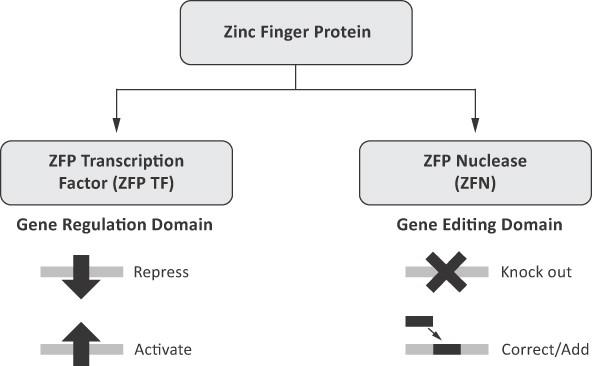
**Schematic of ZFP genome editing and gene regulation ** In contrast, if cells with a mutation in a particular gene are treated not only with ZFNs, but also with a DNA sequence that encodes the correct gene sequence (referred to as a “donor” DNA) and with ZFNs that recognize and bind to sequences flanking the mutation, the cell’s repair machinery can use the donor as a template to correct the mutated gene. This ZFN-mediated gene correction enables the corrected gene to be expressed in its natural chromosomal context and may provide a novel approach for the precise repair of DNA sequence mutations responsible for certain monogenic diseases. In addition to providing a donor sequence that encodes a complete gene, a new copy of a gene can also be precisely added into the genome at a specific location. The ability to precisely place a gene-sized segment of DNA specifically into a pre-determined location in the genome broadens the range of mutations of a gene that can be corrected in a single step. It also reduces the insertional mutagenesis concerns associated with traditional integrating gene replacement approaches such as lentiviruses, in which the insertion of a new corrective copy of the gene typically occurs at random locations in the genome. The company's In Vivo Protein Replacement Platform™, or IVPRP™, in which its ZFN technology is used to insert a gene encoding a therapeutic protein into a location such as the Albumin gene, is an approach that Sangamo BioSciences is investigating for the treatment of hemophilia B (SB-FIX) and LSDs (SB-318 and SB-913), which may potentially provide a single and potentially curative treatment for these diseases.
Sangamo BioSciences is also evaluating ZFP TFs with the potential to control or regulate the expression of a target gene in the desired manner (Figure 3). For instance, attaching an activation domain to a ZFP will cause a target gene to be expressed at enhanced levels, relative to expression in an untreated cell. Alternatively, a repression domain causes the gene to be downregulated or completely turned off. Pursuant to a collaboration agreement with Shire International GmbH, or Shire, Sangamo BioSciences has a preclinical program for Huntington’s disease in which Sangamo BioSciences is evaluating a ZFP TF designed to differentially down regulate the mutated disease-causing Huntingtin, or HTT, gene, while leaving expression of the normal gene unchanged.
ZFPs can be designed to accomplish a range of functions in genome editing and gene regulation.
To date, the company and its partners have designed, engineered and assembled thousands of ZFPs and have tested many of these proteins for their affinity, or tightness of binding to their DNA target, as well as their specificity, or preference for their intended DNA target. Sangamo BioSciences has developed methods for the design, selection and assembly of ZFPs capable of binding to a wide spectrum of DNA sequences and genes. Sangamo BioSciences has linked ZFPs to endonuclease domains to create highly specific ZFNs and to numerous functional domains to create gene-specific ZFP TFs. Sangamo BioSciences has demonstrated the ability of these proteins to enable genome editing or gene regulation, of hundreds of genes in dozens of different cell types and in whole organisms, including non-human primates, or NHPs, mice, rats, rabbits, pigs, fruit flies, worms, zebrafish and yeast, and in plant species including canola and maize. The company and its collaborators have published data from many of these studies in peer-reviewed scientific journals. ZFNs are currently being used to generate transgenic animals and cell lines that have specific genetic modifications that make them useful models of human disease. These high value biologic tools are being used by academic, and biotechnology and pharmaceutical companies for medical research and drug development. The company's preclinical data have been reviewed by advisory bodies such as the National Institute of Health, or NIH, Recombinant Advisory Committee, or RAC, and regulatory bodies such as the U.S. Food and Drug Administration, or FDA, and Sangamo BioSciences has ongoing clinical trials to evaluate the safety and efficacy of ZFNs in humans.
Sangamo BioSciences has employed several strategies for the application of its ZFNs depending on the disease or indication. The company routinely deliver its therapeutics as nucleic acids, either as messenger RNA, or mRNA, or encoded in a viral vector such as AAV that the cell then uses to make the protein form of the ZFN or ZFP TF. The company can deliver ZFNs ex vivo (outside the body) to isolated cells of the blood, such as T cells, in the case of its clinical HIV, cancer immunotherapy and immunology programs, and HSPCs for its programs in HIV and monogenic blood diseases such as beta-thalassemia and SCD. Sangamo BioSciences is also developing ZFPs in which the company deliver its therapeutic proteins in vivo, either systemically (directly into the blood stream) as in its in vivo genome editing programs in hemophilia and LSD, or directly into a specific tissue such as the brain as in its Huntington’s disease program.
ZFPs provide the Opportunity to Develop a New Class of Human Therapeutics
The company believe that its ZFP technology provides a unique and proprietary basis for a broad new class of drugs that have differential technical advantages over small-molecule drugs, protein pharmaceuticals, RNA-based therapeutics, conventional gene therapy approaches and other genome editing platforms, enabling it to develop therapies for a broad range of unmet medical needs.
The company can generate highly specific ZFNs for genome editing and ZFP TFs for gene regulation and have developed multiple delivery strategies to administer these therapeutics, including using mRNA, AAV, adenovirus, plasmid, and lipid nanoparticles. As more genes and DNA sequences are linked to specific diseases, the company believe that the clinical breadth and scope of its ZFP applications will continue to expand.
For example, ZFPs can:
- Enable genome editing and gene regulation strategies to address novel drug targets. Engineered ZFNs enable the efficient disruption, correction or targeted addition of a gene sequence in a very precise fashion, and ZFP TFs enable either repression or activation of a therapeutically relevant gene in a cell. This gives its technology a degree of flexibility. Direct, targeted modification of the genome cannot be achieved using conventional gene therapy approaches, antisense RNA, siRNA, conventional small molecules, antibodies, or other proteins. The company's ZFN genome editing technology, which requires only brief cellular expression of ZFNs, enables the permanent disruption or addition of a therapeutically relevant gene in a highly targeted fashion. For example, its in vivo genome editing strategy enables targeted insertion of a therapeutic gene into the genome of liver cells. This strategy has the potential to provide an extended or life-long clinical benefit in the treatment of monogenic diseases, such as hemophilia, without the risk of washout of therapeutic genes delivered using non-integrating vectors such as AAV, or the potentially deleterious issues related to random insertion of therapeutic genes into the genome by randomly integrating viral vectors such as lentiviral vectors.
- Provide therapeutic solutions for targets that cannot be effectively addressed by existing drug modalities. The sequencing and publication of the human genome and growing information generated by genome-wide association studies have enabled the identification of both genes and regulatory sequences as potential new therapeutic targets. Many of these targets have a direct role in disease processes but cannot be bound or modulated for therapeutic purposes by small molecules, monoclonal antibodies or RNA based therapeutics. Alternative therapeutic approaches are required to modulate the biological activity of these so-called “non-druggable” targets. One such target is the BCL11A erythroid enhancer, a regulatory sequence, which Sangamo BioSciences is disrupting using ZFNs in HSPCs in order to elevate levels of fetal globin. This target is being developed in collaboration with Bioverativ as a therapeutic approach for beta-thalassemia and SCD.
- Provide high specificity and selectivity for targets. ZFNs and ZFP TFs can be designed to act with high specificity. In addition, as there are only two copies of each gene in a cell, there are generally only two targets per cell for ZFNs and ZFP TFs, which means that ZFNs and ZFP TFs need only to be available in the cell to engage a small number of targets, which may reduce the risk of toxicity. In contrast, drugs that act on protein and RNA targets that are naturally present in higher cellular concentrations may need to be administered in higher concentrations. In addition, because of the higher specificity there may be fewer “off-target effects.” Many small molecule and RNA-based approaches either affect multiple targets demonstrating so-called “off-target effects” or may be toxic in the concentrations required to be therapeutically effective.
- Provides a genome editing platform with superior qualities for therapeutic development. Unlike other less developed bacterial-based genome editing platforms, such as CRISPR/Cas9 and TALENS, its proprietary ZFN genome editing technology is based on human proteins that have co-evolved with its complex human genome. The relative complexity of the protein-DNA interaction of its ZFN platform and the ability to engineer the entire protein-DNA interface also gives it the ability to optimize the components of its genome editing technology to drive efficient cutting with specificity. The ZFN-mediated mechanism is optimized for both gene insertion and gene knockout and over years of developing this platform, Sangamo BioSciences has engineered its ZFN proteins to provide maximum design density (1:2 base pairs), giving it the capability to target virtually any sequence of interest and to place a ZFN exactly where the company choose with single gene specificity. This precision is particularly critical for therapeutic gene insertion and correction. Finally, Sangamo BioSciences has an established validated process for rapid development of a ZFN clinical lead and have taken its therapeutics candidates through regulatory review and into human clinical studies where Sangamo BioSciences is able to evaluate both the safety and efficacy of its approach.
Product Pipeline
The company's Product Development Programs
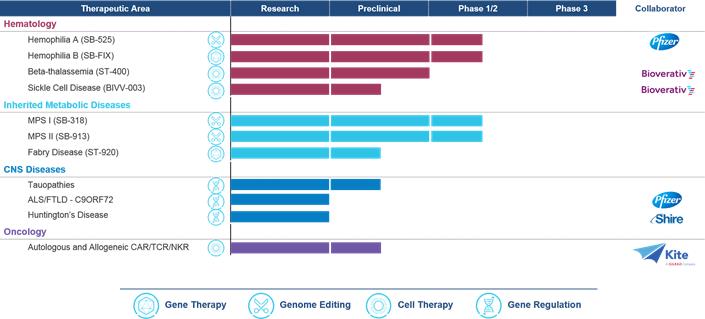
Hemophilia A and B
Hemophilia, a rare bleeding disorder in which the blood does not clot normally. It is also a monogenic disease, or a disease that is caused by a genetic defect in a single gene. There are several types of hemophilia caused by mutations in genes that encode factors which help the blood clot and stop bleeding when blood vessels are injured. Individuals with hemophilia experience bleeding episodes after injuries and spontaneous bleeding episodes that often lead to joint disease such as arthritis. The most severe forms of hemophilia affect males. The standard treatment for individuals with hemophilia is replacement of the defective clotting factor with regular infusion of recombinant clotting factors or plasma concentrates. These therapies are expensive and sometimes stimulate the body to produce antibodies against the factors that inhibit the benefits of treatment. In these situations, other clotting factors such as Factor VII and X may be used to treat patients.
The most prevalent form of the disease, hemophilia A, is caused by a defect in the clotting Factor 8 gene. According to the National Hemophilia Foundation and the World Federation of Hemophilia, hemophilia A occurs in about one in every 5,000 male births in the United States, with approximately 16,000 males currently affected. Defects in clotting Factor 9 gene lead to hemophilia B. Hemophilia B occurs in about one in every 25,000 male births in the United States, with approximately 4,000 males currently affected.
SB-525 – Hemophilia A
Sangamo BioSciences is developing SB-525, a gene therapy product candidate utilizing an AAV carrying a clotting Factor 8 gene construct that is driven by its proprietary synthetic liver specific promoter. In 2016, the company presented preclinical data demonstrating production of supraphysiological levels of human Factor VIII clotting protein, or hFVIII, in mice and NHP. In these dose-ranging preclinical studies, mean hFVIII levels of 5 - 230% of normal were observed using AAV doses in the range of 6.00E+11 – 6.00E+12 vg/kg, the most potent dose response reported in NHPs for a human Factor 8 gene construct at the time.
In 2017, the company initiated a Phase 1/2 clinical trial, the Alta Study, to evaluate the safety and efficacy of SB-525 in adults with severe hemophilia A. The Alta Study is an open-label, ascending-dose study designed to enroll up to 20 adult subjects across six potential dose cohorts. In August 2017, the company announced that the first subject was treated in its Alta Study. The company expect to release preliminary data from the Alta Study by mid-2018.
SB-525 has been granted Orphan Drug and Fast Track designations by FDA as well as Orphan Medicinal Product designation by the European Medicines Agency, or EMA. Sangamo BioSciences is developing SB-525 in collaboration with Pfizer, see “—Collaborations—Pfizer Inc.”
SB-FIX – Hemophilia B
Sangamo BioSciences is developing SB-FIX, an in vivo genome editing product candidate, to treat hemophilia B. Utilizing its ZFN genome editing technology, Sangamo BioSciences is adding a new therapeutic copy of the Factor 9 gene precisely into the Albumin gene locus in liver cells, and using the strong endogenous Albumin promoter to drive expression of the newly inserted gene. The company believe the potential of this approach to provide a permanent correction for a patient may be optimal for a pediatric population by reducing or eliminating the need for chronic infusions of replacement proteins or clotting factor products. Sangamo BioSciences has published data demonstrating the potential utility of this approach for several different monogenic disease applications in addition to hemophilia B.
Preclinical studies of the Albumin genome editing approach have demonstrated that therapeutic levels of Factor IX clotting protein could be generated in a dose-dependent manner in NHPs. There were no significant alterations in circulating Albumin levels. Studies in mice also demonstrated stable Factor IX production for over one year. Preclinical studies in wildtype mice have demonstrated expression of therapeutic levels of human clotting Factor IX protein, or hFIX, from the liver and into the blood for the duration of the 60 week study. Additional preclinical studies in mouse models of hemophilia B demonstrated expression of therapeutic levels of hFIX from the liver and into the blood, which resulted in the correction of the clotting defect in hemophilia B mice treated with a single dose of SB-FIX. SB-FIX was also evaluated in preclinical NHP studies and demonstrated dose-dependent, therapeutic levels of hFIX expression, between 20-50% of normal, in wildtype cynomolgus monkeys, after a single administration of SB-FIX. Levels of hFIX were stable for up to 3 months in treated NHPs. Furthermore, there was a strong dose-response correlation between the level of gene modification at the Albumin locus and the levels of hFIX measured in the blood.
In 2016, the company initiated a Phase 1/2, open-label, ascending dose clinical trial, the FIXtendz Study, to evaluate safety and efficacy of SB-FIX in adult males with severe hemophilia B. The FIXtendz Study is designed to enroll up to 12 subjects across three dose cohorts. In February 2018, the Medicines and Healthcare Products Regulatory Agency, or MHRA, of the United Kingdom granted the Clinical Trial Authorisation, or CTA, for enrollment of subjects into the ongoing Phase 1/2 clinical trial evaluating SB-FIX for hemophilia B. The CTA permits evaluation of SB-FIX in both adults and adolescents. Once preliminary safety and efficacy have been demonstrated in the ongoing SB-FIX Phase 1/2 clinical trial in adults (18 years of older), the company may begin enrolling adolescents (12 - 17 years of age) into the study.
SB-FIX has been granted Orphan Drug and Fast Track designations by the FDA.
Lysosomal Storage Disorders
LSD are a heterogeneous group of rare inherited disorders including: MPS I, MPS II, Fabry disease, Gaucher disease; and many others. These disorders are caused by defects in genes that encode proteins known as enzymes, which break down and eliminate unwanted substances in cells. These enzymes are found in structures called lysosomes which act as recycling sites in cells, breaking down unwanted material into simple products. A defect in a lysosomal enzyme leads to the accumulation of toxic levels of the substance that the enzyme would normally eliminate. These toxic levels may cause cell damage which can lead to serious health problems.
MPS I is caused by mutations in the gene encoding the alpha-L-iduronidase, or IDUA, enzyme, resulting in a deficiency of IDUA enzyme, which is required for the degradation of the glycosaminoglycans, or GAGs, dermatan sulfate and heparin sulfate. The inability to degrade GAGs leads to their accumulation within the lysosomes throughout the body. Individuals with this mutation experience multi-organ dysfunction and damage. Depending on the severity of the mutations and degree of residual enzyme activity, affected individuals may develop enlarged internal organs, joint stiffness, skeletal deformities, corneal clouding, hearing loss and cognition impairments. Three forms of MPS I, in order of increasing severity, include Scheie, Hurler-Scheie and Hurler syndromes. According to the National MPS Society, one in 500,000 births in the United States will result in Scheie syndrome, one in 115,000 births in Hurler/Scheie, and one in 100,000 births results in Hurler syndrome. There are approximately 1,000 MPS I patients in the United States.
MPS II is an X-linked disorder primarily affecting males and caused by mutations in the gene encoding the iduronate-2-sufatase, or IDS, enzyme. This results in a deficiency of IDS enzyme, which is required for the degradation of GAGs. Similar to MPS I, the inability to degrade GAGs leads to their accumulation within the lysosomes throughout the body. Individuals with this mutation experience multi-organ dysfunction and damage. Children with MPS II appear normal at birth but begin showing symptoms of developmental delay by age 2 – 3 years. Depending on the severity of the mutations and degree of residual enzyme activity, affected individuals may develop delayed development, enlarged internal organs, cardiovascular disorders, stunted growth and skeletal abnormalities and hearing loss. The disorder is progressive and symptoms range from mild (normal cognitive function) to severe (cognitively impaired). According to the National MPS Society, one in 100,000 male births in the United States will result in MPS II. There are approximately 500 MPS II patients in the United States.
Fabry disease is an X-linked disorder primarily affecting males and caused by a mutation in the gene encoding the alpha-galactosidase A, or alpha-Gal A, enzyme, resulting in a deficiency of alpha-Gal A enzyme, which is required for the degradation of the ganglioside globotriaosylceramide, a particular type of fatty substance. The inability to degrade this fatty substance leads to its accumulation within the lysosomes throughout the body. Individuals with this mutation experience multi-organ dysfunction and damage. Depending on the severity of the mutations and degree of residual enzyme activity, affected individuals may develop progressive kidney damage, heart attack, stroke, gastrointestinal complications, corneal opacity, tinnitus and hearing loss. Milder forms of the disorder present later in life and affect only the heart or kidneys. According to the National Institutes of Health U.S. National Library of Medicine, one in 40,000 to one in 60,000 male births in the United States will result in Fabry disease. There are approximately 2,200 males with Fabry disease in the United States. This mutation can also occur in females, however is less common and the frequency is unknown.
There are limited treatments currently available for MPS I, MPS II and Fabry disease. For individuals with MPS I, there are only two options: hematopoietic stem cell transplantation, or HSCT, for those with the most severe form of the disease (Hurler) and enzyme replacement therapy, or ERT, for patients with the attenuated forms of the disease (Hurler-Scheie, Scheie). However, the reported mortality rate after HSCT is approximately 15% and the survival rate with successful engraftment is 56%. Most patients with milder forms of the disease receive weekly ERT, usually in a doctor’s office. These IDUA enzyme infusions take on average four to six hours to administer. Weekly and bi-weekly ERT infusions are the only available options for MPS II and Fabry disease, respectively. Because of the availability of few treatment options that effectively and safely treat these diseases, there remains significant unmet medical need.
SB-318 – MPS I
Sangamo BioSciences is developing SB-318, an in vivo genome editing product candidate, to treat MPS I. Using the same approach as its hemophilia B product candidate, SB-FIX, Sangamo BioSciences is adding a new therapeutic copy of the IDUA gene precisely into the Albumin gene locus in the genome of liver cells, using the strong endogenous Albumin promoter to drive expression of the newly inserted gene. The company believe the potential of this approach to provide a permanent correction for a patient may be optimal for a pediatric population by reducing or eliminating the need for chronic ERT infusions.
Preclinical mouse model data demonstrated robust levels of IDUA enzyme expression in the liver, blood plasma and spleen of SB-318 treated mice, resulting in a 10-fold increase in IDUA activity, with sustained elevated levels in the blood plasma over the course of the two month study. Additional preclinical mouse model data demonstrated stable production of therapeutic levels of IDUA enzyme from the liver into the circulation and secondary tissues, including the spleen, lung, muscle, heart and brain, after a single intravenous administration of SB-318. This resulted in the significant reduction of GAG biomarkers in all of the tissues. Behavioral data from Barnes maze tests, collected at the end of the four month study, demonstrated statistically significant preservation of cognitive learning and memory in mice treated with SB-318, compared to untreated mice.
In 2017, the company initiated an open-label, dose-ascending Phase 1/2 clinical trial, the EMPOWERS Study, to evaluate SB-318 in adult subjects with attenuated MPS I. The EMPOWERS Study is designed to enroll up to nine subjects across three ascending dose cohorts. The company expect to present preliminary safety and efficacy data from the EMPOWERS Study in 2018. The company plan to submit a CTA in the first half of 2018 to initiate enrollment of adolescent and pediatric subjects in the United Kingdom into the Phase 1/2 clinical trial.
SB-318 MPS I has been granted Orphan Drug, Rare Pediatric Disease and Fast Track designations by the FDA, as well as Orphan Medicinal Product designation by the EMA.
SB-913 – MPS II
Sangamo BioSciences is developing SB-913, an in vivo genome editing product candidate, to treat MPS II. Similar to SB-318, Sangamo BioSciences is using its ZFN genome editing technology to add a new therapeutic copy of the IDS gene precisely into the Albumin gene locus in the genome of liver cells, using the strong endogenous Albumin promoter to drive expression of the newly inserted gene.
Preclinical mouse model data demonstrated robust levels of IDS enzyme expression in the liver, blood plasma and spleen of SB-913 treated mice, resulting in a 100-fold increase in IDS activity, with sustained elevated levels in the blood plasma over the course of the entire study. Additional preclinical mouse model data demonstrated stable production of therapeutic levels of IDS enzyme from the liver into the circulation and additional secondary tissues, including the spleen, lung, muscle, heart and brain, after a single intravenous administration of SB-913. This resulted in the significant reduction of GAG biomarkers across all the tissues. Behavioral data from Barnes maze tests, collected at the end of the four month study demonstrated statistically significant preservation of cognitive learning and memory in mice treated with SB-913, compared to untreated mice.
In 2017, the company initiated an open-label, dose-ascending Phase 1/2 clinical trial, the CHAMPIONS Study, to evaluate the safety and efficacy of SB-913 in adult male subjects with attenuated MPS II, designed to enroll up to nine subjects across three ascending dose cohorts. In November 2017, the company announced that the first subject had been treated in the CHAMPIONS Study. In February 2018, the company presented preliminary six-week safety data from the first subject enrolled in the CHAMPIONS Study. The data demonstrated that the subject tolerated the infusion well. Mild (Grade 1) adverse events related to the study drug were reported on the fourth day after dosing. These were dizziness, weakness and frequent urination, all of which resolved within one day without treatment. No other adverse events related to the study drug have been observed. Liver function tests have remained within normal limits for the patient since the infusion. The company expect to present additional safety and efficacy data from the EMPOWERS Study by mid 2018. The company plan to submit a CTA in the first half of 2018 to initiate enrollment of adolescent and pediatric subjects in the United Kingdom into the Phase 1/2 clinical trial.
SB-913 has been granted Orphan Drug, Rare Pediatric Disease and Fast Track designations by the FDA, as well as Orphan Medicinal Product designation by the EMA.
ST-920 — Fabry Disease
Sangamo BioSciences is developing ST-920 for Fabry disease, a gene therapy product candidate utilizing an AAV, carrying a galactosidase alpha, or GLA, gene construct, coding for the alpha-Gal A enzyme, driven by its proprietary synthetic liver specific promoter. Sangamo BioSciences is currently conducting IND-enabling studies for ST-920 and expect to file an IND application with the FDA by mid 2018.
Hemoglobinopathies: Beta-thalassemia and Sickle Cell Disease
Mutations in the gene encoding beta-globin, the oxygen carrying protein of red blood cells, lead to hemoglobinopathies such as beta-thalassemia and sickle cell disease, or SCD. Both diseases manifest in the months after birth, when patients switch from producing functional fetal gamma-globin to a mutant form of adult beta-globin, which results in their condition. Naturally occurring increased levels of fetal hemoglobin have been shown to reduce the severity of both beta-thalassemia and SCD.
Beta-thalassemia is a rare disorder that results in greatly impaired production of healthy red blood cells despite bone marrow over activity, leading to life-threatening anemia, enlarged spleen, liver and heart, and bone abnormalities. Sangamo BioSciences is focused on Beta-thalassemia major which is a severe form of thalassemia that requires regular, often monthly, blood transfusions and subsequent iron-chelation therapy to treat iron overload. The Centers for Disease Control and Prevention, or CDC, estimates that 1,000 people have beta-thalassemia major in the United States, and an unknown number carry the genetic trait and can pass it on to their children.
In SCD, the mutation causes the red blood cells to form an abnormal sickle or crescent shape. The cells are fragile and deliver less oxygen to the body’s tissues. They can also get stuck more easily in small blood vessels and break into pieces that can interrupt healthy blood flow which further decrease the amount of oxygen flowing to body tissues. Almost all patients with SCD experience these painful vaso-occlusive crises, which can last from hours to days and may cause irreversible organ damage. Current standard of care is to manage and control symptoms, and to limit the number of crises. Treatments include administration of hydroxyurea, blood transfusions, iron-chelation therapy, pain medications and antibiotics. The CDC estimates that there are 90,000 to 100,000 Americans living with SCD, which occurs in approximately 1 out of every 365 African-American births and 1 out of every 16,300 Hispanic-American births.
ST-400 – Beta-thalassemia; BIVV-003 — SCD
Sangamo BioSciences is developing ST-400 for the treatment of beta-thalassemia and its collaboration partner, Bioverativ, is developing BIVV-003 for the treatment of SCD. Both ST-400 and BIVV-003 are genome-edited cell therapies that use its ZFN genome editing technology to modify a patient’s own, or autologous, HSPCs to produce functional red blood cells using fetal hemoglobin. The company's genome editing technology can be used in HSPCs to precisely disrupt regulatory sequences that control the expression of key transcriptional regulators, such as the BCL11A erythroid enhancer sequence, to reverse the switch from expression of the mutant adult beta-globin back to the production of functional fetal gamma-globin.
The current standard of care for beta-thalassemia includes chronic blood transfusions, while the standard of care for SCD is a bone marrow transplant, or BMT, of HSPCs from a “matched” related donor, or an allogeneic BMT. However, these therapies are limited due to the risk of iron overload with blood transfusions, requiring subsequent iron chelation therapy, and the scarcity of matched donors and the significant risk of Graft versus Host Disease, or GvHD, with BMTs after transplantation of the foreign cells. By performing genome editing in HSPCs that are isolated from and subsequently returned to the same patient (i.e., an autologous HSPC transplant), its approach has the potential to address these limitations. The goal of this approach is to develop a one-time long-lasting treatment for beta-thalassemia and SCD.
Preclinical data from clinical-scale in vitro studies have demonstrated that ST-400 and BIVV-003 can be manufactured by reproducible, high-level, ZFN-mediated modification in HSPCs mobilized in peripheral blood at clinical production scale (>108 cells), with an on-target modification efficiency of greater than 80%. Furthermore, erythroid differentiation of enhancer targeted cells showed modification of both BCL11A erythroid enhancer alleles in more than 50% of the erythroid colonies and resulted in a greater than four-fold increase in gamma globin mRNA and protein production, compared to controls. Specificity studies of ST-400 and BIVV-003 revealed no detectable off-target activity using state-of-the art, unbiased, highly sensitive oligo-capture assays. Preclinical data from in vivo studies in immune-deficient mice demonstrated robust long-term (19 weeks) engraftment and that targeted gene modification was maintained through multi-lineage differentiation in the bone marrow and peripheral blood.
The company's IND for ST-400 was cleared by the FDA in September 2017, and Sangamo BioSciences has designed an open-label, single arm Phase 1/2 clinical trial to evaluate the safety and efficacy of ST-400 in up to 6 adult subjects with beta-thalassemia. The company expect to initiate this trial in early 2018.
Bioverativ is its partner for ST-400 and is responsible for the clinical development of BIVV-003 for SCD. For more information relating to its collaboration with Bioverativ, see “—Collaborations—Bioverativ.”
CNS-Tauopathies
Sangamo BioSciences is using its ZFP-TF gene regulation platform to develop potential gene therapies for tauopathy disorders, including Alzheimer’s disease and other neurodegenerative diseases. The company believe a reduction in tau protein levels can help reduce intracellular tau protein aggregation and the formation of neurofibrillary tangles in neurons, potentially ameliorating or reversing disease progression. The company believe this approach may have a significant advantage compared to monoclonal antibody-based approaches to Alzheimer’s disease and other tauopathy disorders because it is designed to selectively down-regulate the tau gene in neurons with the goal of reducing all forms of the tau protein globally across the CNS. In contrast, monoclonal antibody-based approaches are limited in that they can only bind to certain forms of tau proteins.
Preclinical studies in wildtype mice demonstrated that a single administration of tau-targeting ZFP-TFs resulted in up to 70% reduction of tau mRNA and protein expression across the entire CNS, as well as sustained and well-tolerated ZFP-TF expression with minimal impact on inflammatory markers. Additional preclinical studies in amyloid mouse models of Alzheimer’s disease demonstrated up to 80% reduction of tau protein levels in the brain and cerebrospinal fluid, as well as significantly reduced neuritic dystrophy after a single administration of ZFP-TFs in mice with established disease pathology.
Sangamo BioSciences is currently conducting preclinical studies in NHPs to evaluate its ZFP-TFs in larger mammalian species. The company intend to seek a partner with disease area expertise for the clinical development and commercialization of this program.
C9ORF72–linked ALS/FTLD
In December 2017, the company entered into a research collaboration and license agreement with Pfizer to develop and commercialize gene therapy products that use its ZFP TFs to treat ALS and FTLD linked to mutations of the C9ORF72 gene. ALS and FTLD are part of a spectrum of neurodegenerative disorders caused by mutations in the C9ORF72 gene that involve hundreds of additional repetitions of a six base pair sequence of DNA. This ultimately leads to the deterioration of motor neurons, in the case of ALS, or neurons in the frontal and temporal lobes, in the case of FTLD. Currently, there are no cures to halt or reverse the progression of ALS or FTLD. The C9ORF72 mutation is linked to approximately one-third of cases of familial ALS. The company and Pfizer plan to investigate allele-specific ZFP-TFs with the potential to differentiate the mutant C9ORF72 allele from the wildtype allele and to specifically down-regulate expression of the mutant form of the gene.
The company also have research stage programs in other monogenic diseases, immunology and cancer immunotherapy. See “—Collaborations—Pfizer Inc.,” for more information relating to this agreement.
Huntington’s Disease
Huntington’s disease is an inherited, progressive neurologic disease for which there is no treatment or cure. The disease is caused by a particular type of mutation in a single gene, the HTT gene. Most patients inherit one normal and one defective or mutant copy of the HTT gene, which causes Huntington’s disease. The mutation is characterized by expansion of a repeated stretch of DNA sequence within the gene called a “CAG repeat.” A normal copy of the HTT gene usually has 10 to 29 of these CAG repeats but a defective copy has many more—generally greater than 39 repeats. While the protein produced by the normal copy of the gene appears to be essential for development (mice lacking the gene do not survive to birth), the product of the mutated gene is damaging to cells. Symptoms, which include deterioration of muscle control, cognition and memory, usually develop between 35 and 44 years of age. It is known that the greater the number of CAG repeats, the earlier the onset. Huntington’s disease is usually fatal within 15 to 20 years after the onset of symptoms. The disease has a high prevalence for an inherited disorder. According to the Huntington’s Disease Society of America, approximately 30,000 people in the United States have Huntington’s disease. In addition, it is estimated that approximately 200,000 people in the United States are at risk of developing the disease.
Research in animal models of the disease has shown that lowering the levels of the mutant HTT protein can prevent, or even reverse, disease progression. However, to date most “HTT-lowering” methods decrease levels of both the normal and mutant forms of HTT, raising potential safety concerns given the importance of normal HTT protein. In collaboration with Shire, Sangamo BioSciences is developing ZFP TFs that can selectively repress the expression of the mutant disease-causing form of HTT while leaving expression levels of the normal gene unchanged. Preclinical studies in animal models of the disease are ongoing and Shire is responsible for all clinical development activities including filing the IND application. For more information on its collaboration with Shire, see “—Collaborations—Shire International GmbH.”
Legacy Clinical Research Programs
Human Immunodeficiency Virus, or HIV, and Acquired Immunodeficiency Syndrome, or AIDS
HIV infection results in the death of immune system cells, particularly CD4+ T-cells, and thus leads to AIDS, a condition in which the body’s immune system is depleted to such a degree that the patient is unable to fight off common infections. Ultimately, these patients succumb to opportunistic infections or cancers. According to the most recent data from the CDC, it is estimated that there were 1.0 million people living with HIV/AIDS in the United States in 2015, and there are now over 36.7 million people living with HIV and AIDS worldwide.
Current Treatments and Unmet Medical Need
Currently, there are over 30 antiretroviral drugs approved by the FDA to treat people infected with HIV. While these drugs can suppress virus in the blood to undetectable levels, they cannot eliminate the reservoir of cells containing genomically-integrated HIV from the body. Hence, individuals infected with HIV need to take antiretroviral drugs continuously. The drugs are expensive and can have significant side effects over time. There is no therapeutic approach available that protects CD4+ T-cells, suppresses viral load, reduces the viral reservoir and does not require daily dosing.
SB-728 – HIV/AIDS
SB-728 uses its ZFN-mediated genome editing technology to disrupt the CCR5 gene in cells of a patient’s immune system to make these cells permanently resistant to HIV infection. CCR5 is a co-receptor for HIV entry into T-cells and if CCR5 is not expressed on the cell surface HIV cannot infect them or infects them with lower efficiency. The aim of this approach is to provide the patient with a population of HIV-resistant cells that can fight HIV and opportunistic infections, by mimicking the naturally occurring CCR5 delta-32 mutation that renders a population of individuals largely resistant to infection by the most common strains of HIV. Sangamo BioSciences is evaluating this genome editing approach to disrupt the CCR5 gene in both T cells and HSPCs as two potential therapeutic candidates, SB-728-T and SB-728-HSPC, respectively.
Sangamo BioSciences has conducted several clinical trials with SB-728-T, which were designed to evaluate safety and tolerability of SB-728-T, as well as the effect of SB-728-T on subjects’ CD4 T-cell counts, levels of CCR5-modified T-cells, viral burden during a treatment interruption (TI) from anti-retroviral therapy, or ART, and measure of the viral reservoir. The data to date have demonstrated an ability to efficiently knock out the CCR5 gene in T-cells by ZFN-driven genome editing and grow the cells ex vivo, that a single infusion of SB-728-T led to proven engraftment, expansion and persistence of T-cells in vivo, sustained increases in CD4 T-cell counts, a significant and continuous decay of the HIV reservoir and the ability of certain subjects to control their viral loads for prolonged periods in the absence of ART. Over 100 subjects have been treated to date and the treatment appears to be well-tolerated.
In addition, Sangamo BioSciences has an ongoing investigator-sponsored Phase 1/2 clinical trial (SB-728mR-HSPC) to investigate SB-728-HSPC as a self-renewable and potentially lifelong source of HIV-resistant immune cells.
The company plan to advance the SB-728 program through externally-funded collaborations.
Collaborations
Sangamo BioSciences has established collaborative and strategic partnerships for several of its therapeutic programs and also for several non-therapeutic applications of its technology. The company will continue to pursue further partnerships when appropriate with selected pharmaceutical and biotechnology to fund internal research and development activities and to assist in product development and commercialization. Sangamo BioSciences is applying its ZFN technology platform to several commercial applications in which its products provide it and its strategic partners and collaborators with potential technical, competitive and economic advantages.
Kite Pharma, Inc.
In February 2018, the company entered into a collaboration and license agreement with Kite, a wholly-owned subsidiary of Gilead, for the research, development and commercialization of potential engineered cell therapies for cancer. Kite will be responsible for all clinical development and commercialization of any resulting products. Except for confidentiality obligations and certain representations, warranties and covenants, which are effective upon execution, the effectiveness of the Kite agreement is subject to the expiration or termination of all applicable waiting periods under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and other customary closing conditions.
Subject to the terms of this agreement, the company will, upon effectiveness of this agreement, grant Kite an exclusive, royalty-bearing, worldwide, sublicensable license, under its relevant patents and know-how, to develop, manufacture and commercialize, for the purpose of treating cancer, specific cell therapy products that may result from the research program and that are engineered ex vivo using selected ZFNs and AAVs developed under the research program, to express CARs, TCRs or NKRs directed to candidate targets.
During the research program term and subject to certain exceptions, except pursuant to this agreement, the company will be prohibited from researching, developing, manufacturing and commercializing, for the purpose of treating cancer, any cell therapy product that, as a result of ex vivo genome editing, expresses a CAR, TCR or NKR that is directed to a target expressed on or in a human cancer cell. After the research program term concludes and subject to certain exceptions, except pursuant to this agreement, the company will be prohibited from developing, manufacturing and commercializing, for the purpose of treating cancer, any cell therapy product that, as a result of ex vivo genome editing, expresses a CAR, TCR or NKR that is directed to a candidate target.
Upon the effectiveness of this agreement, the company will receive a $150 million upfront payment from Kite. In addition, Kite will reimburse its direct costs to conduct the joint research program, and Kite will be responsible for all subsequent development, manufacturing and commercialization of any licensed products. Sangamo BioSciences is also eligible to receive contingent development- and sales-based milestone payments that could total up to $3.01 billion if all of the specified milestones set forth in this agreement are achieved. Of this amount, approximately $1.26 billion relates to the achievement of specified research, clinical development, regulatory and first commercial sale milestones, and approximately $1.75 billion relates to the achievement of specified sales-based milestones if annual worldwide net sales of licensed products reach specified levels. Each development- and sales-based milestone payment is payable ![]() only once for each licensed product, regardless of the number of times that the associated milestone event is achieved by such licensed product, and (ii) only for the first ten times that the associated milestone event is achieved, regardless of the number of licensed products that may achieve such milestone event. In addition, the company will be entitled to receive escalating, tiered royalty payments with a percentage in the single digits based on potential future annual worldwide net sales of licensed products. These royalty payments will be subject to reduction due to patent expiration, entry of biosimilar products to the market and payments made under certain licenses for third-party intellectual property.
only once for each licensed product, regardless of the number of times that the associated milestone event is achieved by such licensed product, and (ii) only for the first ten times that the associated milestone event is achieved, regardless of the number of licensed products that may achieve such milestone event. In addition, the company will be entitled to receive escalating, tiered royalty payments with a percentage in the single digits based on potential future annual worldwide net sales of licensed products. These royalty payments will be subject to reduction due to patent expiration, entry of biosimilar products to the market and payments made under certain licenses for third-party intellectual property.
Kite has the right to terminate this agreement, in its entirety or on a per licensed product or per candidate target basis, for any reason after a specified notice period. Each party has the right to terminate this agreement on account of the other party’s bankruptcy or material, uncured breach.
Pfizer Inc.
Sangamo BioSciences has two separate collaboration agreements with Pfizer Inc., or Pfizer. In May 2017, the company entered into an exclusive, global collaboration and license agreement with Pfizer, pursuant to which the company established a collaboration for the research, development and commercialization of SB-525, its gene therapy product candidate for hemophilia A, and closely related products.
Under this agreement, Sangamo BioSciences is responsible for conducting the Phase 1/2 clinical trial and certain manufacturing activities for SB-525, while Pfizer is responsible for subsequent worldwide development, manufacturing, marketing and commercialization of SB-525. The company may also collaborate in the research and development of additional AAV-based gene therapy products for hemophilia A.
The company received an upfront fee of $70.0 million and are eligible to receive development milestone payments contingent on the achievement of specified clinical development, intellectual property, regulatory and first commercial sale milestones for SB-525 and potentially other products. The total amount of potential clinical development, intellectual property, regulatory, and first commercial sale milestone payments, assuming the achievement of all specified milestones in this agreement, is $475.0 million, which includes up to $300.0 million for SB-525 and up to $175.0 million for other products that may be developed under the agreement, subject to reduction on account of payments made under certain licenses for third party intellectual property. In addition, Pfizer agreed to pay it royalties for each potential licensed product developed under the agreement that are an escalating tiered, double-digit percentage of the annual net sales of such product and are subject to reduction due to patent expiration, entry of biosimilar products to the market and payment made under certain licenses for third party intellectual property.
Subject to the terms of the agreement, the company granted Pfizer an exclusive, worldwide, royalty-bearing license, with the right to grant sublicenses, to use certain technology controlled by it for the purpose of developing, manufacturing and commercializing SB-525 and related products. Pfizer granted it a non-exclusive, worldwide, royalty free, fully paid license, with the right to grant sublicenses, to use certain manufacturing technology developed under the agreement and controlled by Pfizer to manufacture its products that utilize the AAV delivery system. During a specified period, neither the company nor Pfizer will be permitted to clinically develop or commercialize, outside of the collaboration, certain AAV-based gene therapy products for hemophilia A.
Unless earlier terminated, the agreement has a term that continues, on a per product and per country basis, until the later of ![]() the expiration of patent claims that cover the product in a country, (ii) the expiration of regulatory exclusivity for a product in a country, and (iii) fifteen years after the first commercial sale of a product in a country. Pfizer has the right to terminate the agreement without cause in its entirety or on a per product or per country basis. The agreement may also be terminated by either party based on an uncured material breach by the other party or the bankruptcy of the other party. Upon termination for any reason, the license granted by it to Pfizer to develop, manufacture and commercialize SB-525 and related products will automatically terminate. Upon termination by it for cause or by Pfizer any country or countries, Pfizer will automatically grant it an exclusive, royalty-bearing license under certain technology controlled by Pfizer to develop, manufacture and commercialize SB-525 in the terminated country or countries.
the expiration of patent claims that cover the product in a country, (ii) the expiration of regulatory exclusivity for a product in a country, and (iii) fifteen years after the first commercial sale of a product in a country. Pfizer has the right to terminate the agreement without cause in its entirety or on a per product or per country basis. The agreement may also be terminated by either party based on an uncured material breach by the other party or the bankruptcy of the other party. Upon termination for any reason, the license granted by it to Pfizer to develop, manufacture and commercialize SB-525 and related products will automatically terminate. Upon termination by it for cause or by Pfizer any country or countries, Pfizer will automatically grant it an exclusive, royalty-bearing license under certain technology controlled by Pfizer to develop, manufacture and commercialize SB-525 in the terminated country or countries.
In December 2017, the company entered into a separate exclusive, global collaboration and license agreement with Pfizer for the development and commercialization of potential gene therapy products that use ZFP-TFs to treat ALS and FTLD linked to mutations of the C9ORF72 gene. Pursuant to this agreement, the company agreed to work with Pfizer on a research program to identify, characterize and preclinically develop ZFP-TFs that bind to and specifically reduce expression of the mutant form of the C9ORF72 gene.
The company received a $12.0 million upfront payment from Pfizer and are eligible to receive up to $60.0 million in development milestone payments from Pfizer contingent on the achievement of specified preclinical development, clinical development and first commercial sale milestones, and up to $90.0 million commercial milestone payments if annual worldwide net sales of the licensed products reach specified levels. In addition, Pfizer will pay it royalties based on an escalating tiered, mid- to high-single digit percentage of the annual worldwide net sales of the licensed products. These royalty payments are subject to reduction due to patent expiration, entry of biosimilar products to the market and payments made under certain licenses for third party intellectual property. Each party will be responsible for the cost of its performance of the research program. Pfizer will be operationally and financially responsible for subsequent development, manufacturing and commercialization of the licensed products.
Subject to the terms of the agreement, the company granted Pfizer an exclusive, royalty-bearing, worldwide, license under its relevant patents and know-how to develop, manufacture and commercialize gene therapy products that use resulting ZFP-TFs that satisfy pre-agreed criteria. During a specified period, neither its company nor Pfizer will be permitted to research, develop, manufacture or commercialize outside of the collaboration any zinc finger proteins that specifically bind to the C9ORF72 gene.
Unless earlier terminated, the agreement has a term that continues, on a per licensed product and per country basis, until the later of ![]() the expiration of patent claims that cover the licensed product in a country, (ii) the expiration of regulatory exclusivity for a licensed product in a country, and (iii) fifteen years after the first commercial sale of a licensed product in a major market country. Pfizer also has the right to terminate the agreement without cause in its entirety or on a per product or per country basis. The agreement may also be terminated by either party based on an uncured material breach by the other party or the bankruptcy of the other party. The agreement will also terminate if Sangamo BioSciences is unable to identify any lead candidates for development within a specified period of time or if Pfizer elects not to advance a lead candidate beyond a certain development milestone within a specified period of time. Upon termination for any reason, the license granted by it to Pfizer to develop, manufacture and commercialize licensed products under the agreement will automatically terminate. Upon termination by it for cause or by Pfizer without cause for any licensed product or licensed products in any country or countries, the company will have the right to negotiate with Pfizer to obtain a non-exclusive, royalty-bearing license under certain technology controlled by Pfizer to develop, manufacture and commercialize the licensed product or licensed products in the terminated country or countries.
the expiration of patent claims that cover the licensed product in a country, (ii) the expiration of regulatory exclusivity for a licensed product in a country, and (iii) fifteen years after the first commercial sale of a licensed product in a major market country. Pfizer also has the right to terminate the agreement without cause in its entirety or on a per product or per country basis. The agreement may also be terminated by either party based on an uncured material breach by the other party or the bankruptcy of the other party. The agreement will also terminate if Sangamo BioSciences is unable to identify any lead candidates for development within a specified period of time or if Pfizer elects not to advance a lead candidate beyond a certain development milestone within a specified period of time. Upon termination for any reason, the license granted by it to Pfizer to develop, manufacture and commercialize licensed products under the agreement will automatically terminate. Upon termination by it for cause or by Pfizer without cause for any licensed product or licensed products in any country or countries, the company will have the right to negotiate with Pfizer to obtain a non-exclusive, royalty-bearing license under certain technology controlled by Pfizer to develop, manufacture and commercialize the licensed product or licensed products in the terminated country or countries.
Following termination by it for Pfizer’s material breach, Pfizer will not be permitted to research, develop, manufacture or commercialize ZFPs that specifically bind to the C9ORF72 gene for a period of time. Following termination by Pfizer for its material breach, the company will not be permitted to research, develop, manufacture or commercialize ZFPs that specifically bind to the C9ORF72 gene for a period of time.
Bioverativ Inc.
Sangamo BioSciences is party to an exclusive worldwide collaboration and license agreement with Bioverativ to develop therapeutics for hemoglobinopathies, focused on beta-thalassemia and SCD. Under the agreement, Sangamo BioSciences is jointly conducting two research programs: the beta-thalassemia program and the SCD program. In the beta-thalassemia program, Sangamo BioSciences is responsible for all discovery, research and development activities through the first human clinical trial. In the SCD program, both parties are responsible for research and development activities through the submission of an IND application for ZFP therapeutics intended to treat SCD. Bioverativ reimburses it for agreed upon internal and external program-related costs.
Under both programs, Bioverativ is responsible for subsequent worldwide clinical development, manufacturing and commercialization of licensed products developed under the agreement. At the end of the specified research terms for each program or under certain specified circumstances, Bioverativ has the right to step in and take over any of its remaining activities. Furthermore, Sangamo BioSciences has an option to co-promote in the United States any licensed products to treat beta-thalassemia and SCD developed under the agreement, and Bioverativ will compensate it for such co-promotion activities. Subject to the terms of the agreement, Sangamo BioSciences has granted Bioverativ an exclusive, royalty-bearing license, with the right to grant sublicenses, to use certain ZFP and other technology controlled by it for the purpose of researching, developing, manufacturing and commercializing licensed products developed under the agreement. Sangamo BioSciences has also granted Bioverativ a non-exclusive, worldwide, royalty-free, fully paid license, with the right to grant sublicenses, under its interest in certain other intellectual property developed pursuant to the agreement. During the term of the agreement, Sangamo BioSciences is not permitted to research, develop, manufacture or commercialize, outside of the agreement, certain gene therapy products that target genes relevant to the licensed products.
Under the agreement, the company received an upfront license fee of $20.0 million and are eligible to receive development and sales milestone payments upon the achievement of specified regulatory, clinical development and sales milestones. The total amount of potential regulatory, clinical development, and sales milestone payments, assuming the achievement of all specified milestones in the agreement, is $276.3 million. In addition, the company will receive royalty payments for each licensed product that are a tiered double-digit percentage of annual net sales of each product.
The agreement may be terminated by ![]() it or Bioverativ for the uncured material breach of the other party, (ii) it or Bioverativ for the bankruptcy or other insolvency proceeding of the other party; (iii) Bioverativ, upon 180 days’ advance written notice to it and (iv) Bioverativ, for certain safety reasons upon written notice to, and after consultation with, it. As a result, actual future milestone payments could be lower than the amounts stated above.
it or Bioverativ for the uncured material breach of the other party, (ii) it or Bioverativ for the bankruptcy or other insolvency proceeding of the other party; (iii) Bioverativ, upon 180 days’ advance written notice to it and (iv) Bioverativ, for certain safety reasons upon written notice to, and after consultation with, it. As a result, actual future milestone payments could be lower than the amounts stated above.
Shire International GmbH
Sangamo BioSciences is party to a collaboration and license agreement with Shire International GmbH, or Shire, to research, develop and commercialize a ZFP therapeutic for treating Huntington’s disease. The company received an upfront license fee of $13.0 million. Shire does not have any milestone payment obligations, but is required to pay single digit percentage royalties to it, up to a specified maximum cap, on the commercial sales of therapeutic products for Huntington’s disease. Sangamo BioSciences is required to pay single digit percentage royalties to Shire, up to a specified maximum cap, on commercial sales of therapeutic products from programs returned under the original agreement (which include blood clotting Factors VIII and IX) that use two zinc fingers.
Pursuant to the agreement, the company granted Shire an exclusive, world-wide, royalty-bearing license, with the right to grant sublicenses, to use its ZFP technology for the purpose of developing and commercializing human therapeutic and diagnostic products for the HTT gene. During the term of the agreement, Sangamo BioSciences is not permitted to research, develop or commercialize, outside of the agreement, certain products that target the HTT gene. The company satisfied the deliverables and research services responsibilities within the amended arrangement which were completed in 2017. The agreement may be terminated by ![]() it or Shire, in whole or in part, for the uncured material breach of the other party, (ii) it or Shire for the bankruptcy or other insolvency proceeding of the other party and (iii) Shire, in its entirety, effective upon at least 90 days’ advance written notice.
it or Shire, in whole or in part, for the uncured material breach of the other party, (ii) it or Shire for the bankruptcy or other insolvency proceeding of the other party and (iii) Shire, in its entirety, effective upon at least 90 days’ advance written notice.
Other Partnerships
In addition to its partnerships for the development of human therapeutic applications, Sangamo BioSciences has also licensed its technology in several other areas, such as plant agriculture and research reagents, including the production of transgenic animals and cell-line engineering. These license partners include Dow AgroSciences LLC, Sigma-Aldrich Corporation, Genentech, Inc., Open Monoclonal Technology, Inc. and F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc.
Intellectual Property
Patents and licenses are important to its business. The company's strategy is to file or license patent applications to protect technology, inventions and improvements to inventions that the company consider important for the development of its genome editing and gene regulation technology. The company seek patent protection and licenses that relate to its technology and candidates in its pipeline and/or may be important to its future. Sangamo BioSciences has filed numerous patents and patent applications with the United States Patent and Trademark Office, or U.S. PTO, and foreign jurisdictions. This proprietary intellectual property includes methods relating to the design of zinc finger, Transcription activator-like effector, or TALE, proteins and Clustered Regularly Interspaced Short Palindromic Repeats, or CRISPR/Cas editing systems, therapeutic applications of genome editing technology, enabling technologies related to its platform and the use of genome editing across a variety of applications. The company rely on a combination of patent, copyright, trademark, proprietary know–how, continuing technological innovations, trade secret laws, as well as confidentiality agreements, materials transfer agreements, research agreements and licensing agreements, to establish and protect its proprietary rights.
In-Licensed Technology
Sangamo BioSciences has licensed intellectual property directed to the design, selection, and use of ZFPs, ZFNs and ZFP TFs for genome editing and gene regulation from the Massachusetts Institute of Technology, Johnson & Johnson, The Scripps Research Institute, the California Institute of Technology and the University of Utah. These licenses grant it rights to make, use and sell ZFPs, ZFNs and ZFP TFs under 9 families of patent filings. As of February 15, 2018, these patent filings have resulted in over 10 issued U.S. patents and over 40 granted foreign patents are still active, with 3 currently pending U.S. patent applications and 8 pending applications in foreign patent offices. Sangamo BioSciences has non-exclusive licenses from the NIH for intellectual property related to the composition of the AAV5 vector and to methods of production of AAV, both of which will expire in 2021.
The company entered into a patent license agreement with the Massachusetts Institute of Technology, or MIT, in May 1996, as subsequently amended, whereby MIT granted it a worldwide exclusive license to technology and patents relating to the design, selection and use of ZFPs for all fields of use, including the right to sublicense. Under the patent license agreement, Sangamo BioSciences is obligated to pay an annual license fee, low single-digit royalties of product sales, sublicense issuance fees and annual sublicense maintenance fees, a percentage of its sublicense revenues, and milestone payments upon achievement of certain commercial development milestones. The aggregate milestone payments under the patent license agreement are $450,000, of which $150,000 has been paid. At its request, the patent license has been amended to return some of the intellectual property that was added via amendment after the original agreement was put in place. This does not affect the development of its technology. The patent license agreement still expires upon the expiration of the last patent covered by the remainder of patents under the license agreement. Based on currently licensed patents, the patent license agreement will expire in October 2019. MIT may terminate the license agreement upon a material default by it that remains uncured following written notice. The company may terminate the license agreement at any time upon six months’ written notice.
The company entered into a sublicense agreement with Johnson & Johnson in May 1996, whereby Johnson & Johnson granted it a worldwide exclusive sublicense to technology and patents for the research, development and commercialization of human and animal therapeutic and diagnostic products using engineered ZFPs, including the right to sublicense. These patents were originally exclusively licensed by Johnson & Johnson from The Scripps Research Institute. Under the sublicense agreement, the company will pay low single-digit royalty payments based upon sales of license products by it or its sublicensees and a milestone payment upon the achievement of a commercial development milestone. The sublicense agreement expires upon the expiration of the last patent covered by the sublicense agreement. Based on currently issued patents and currently filed patent applications, the sublicense agreement will expire on or about June 5, 2018. Johnson & Johnson has the right to terminate the sublicense agreement upon a breach or default by it that remains uncured following written notice of such default. The company may terminate the sublicense agreement at any time upon sixty days’ written notice.
The company entered into a license agreement with The Scripps Research Institute in March 2000, as subsequently amended, whereby The Scripps Research Institute granted it a worldwide exclusive license to technology and patents for the research, development and commercialization of products and services using engineered ZFPs, excluding the use of these engineered ZFPs in plant agriculture, therapeutics and diagnostics. Under the license agreement, Sangamo BioSciences is required to pay a low-single digit royalty on sales of licensed products by it and its sublicensees, subject to an annual minimum. The license agreement expires upon the expiration of the last patent covered by the license agreement. Based on currently issued patents and currently filed patent applications, the license agreement will expire on or about May 27, 2018. Each party may terminate the license agreement upon a material default by the other party that remains uncured following written notice.
The company entered into a license agreement with California Institute of Technology, or CalTech, in November 2003, as subsequently amended, whereby CalTech granted it a worldwide exclusive license to certain patents related to chimeric nucleases for genome targeting for all fields of use, including the right to sublicense. In consideration of the license grant, the company issued certain shares of its common stock to CalTech, but have no obligations to make milestone or royalty payments to CalTech. The license agreement expires upon the expiration of the intellectual property rights licensed to it. Based on currently issued patents and currently filed patent applications, the license agreement will expire in September 2023. Each party may terminate the license agreement upon a material default by the other party that remains uncured following written notice. The company may terminate the agreement at any time upon 30 days written notice.
The company entered into a patent license agreement with the University of Utah Research Foundation, in September 2004, as subsequently amended, whereby Utah granted it a worldwide license to technology and patents relating to the use of ZFNs for all fields of use, including the right to sublicense. Under the patent license agreement, Sangamo BioSciences is obligated to pay an annual license fee, low single-digit royalties of product sales, sublicense issuance fees and annual sublicense maintenance fees, a percentage of its sublicense revenues, and milestone payments upon achievement of certain commercial development milestones. The license agreement expires on the expiration of the last patent covered by the patent license agreement. Based on currently issued patents, the license agreement will expire in March 2025. Utah may terminate the license agreement upon a default by it that remains uncured following written notice. The company may terminate the agreement at any time upon 90 days written notice.
Sangamo BioSciences has entered into licenses potentially useful for specific therapeutic uses of its genome editing technologies with the Regents of the University of California and the Children's Medical Center Corporation. The patents included in these licenses relate to CNS disorders and hemoglobinopathies, respectively. These licenses include 2 patent families, including 3 issued U.S. patents, over 20 pending foreign patents and 2 pending U.S. patents.
Intellectual Property
In addition to its in-licensed patent portfolio, as of February 15, 2018, the company had over 150 families of owned or co-owned patent filings, over 180 issued U.S. patents, over 600 granted foreign patents, over 110 pending U.S. patent applications and over 490 pending foreign patent applications. These patent filings are directed to the design, composition and use of ZFPs, ZFNs, ZFP TFs, TALE proteins and CRISPR/Cas systems and other technology related to its programs.
Some of the earliest zinc finger patents in its portfolio began expiring in 2015, with the average expiration of its currently issued patents expiring being late-2026. However, Sangamo BioSciences has continued to build on this patent portfolio and have been issued additional patents and have applications pending that provide protection for its ZFP technology. These patents in its portfolio may be subject to Patent Term Adjustment (due to delays in patent prosecution by the USPTO), Patent Term Extension (due to review of a patented product by a regulatory agency) or terminal disclaimer. Additionally, patents that may be issued from its pending applications will extend the patent exclusivity of its patent estate. Accordingly, all dates given above for patent expirations are estimates and the actual dates of expirations may differ.
The company believe that its licensed patents and patent applications, as well as its issued patents and pending patent applications, in the aggregate, will provide it with a substantial intellectual property position in its commercial development of its genome editing, gene therapy, cell therapy and gene regulation programs. In this regard, patents issued to it, applied for by it, or exclusively and non-exclusively licensed to it, cover the following types of inventions, processes and products:
- ZFP and ZFN design, engineered nucleases, and compositions: includes DNA target site selection and zinc finger binding domain design and nuclease domain design, DNA nickases, target site arrays, ZFP libraries databases and methods of construction, as well as methods to increase zinc finger binding specificity, nuclease, linker designs (see newly issued US9567609) and methods of making modified plant ZFPs;
- ZFP targeted regulation of endogenous genes: methods relating to activation and inhibition of endogenous cellular genes, identification of accessible regions within chromatin, and regulation of endogenous plant genes;
- ZFP Therapeutics: Treatment of Huntington’s disease (see U.S. patent publication US2017-0096460), cancer therapeutics, treatment of head and neck cancer, glioblastoma, modulation of cardiac contractility and methods to regulate the glucocorticoid receptor, treatments for HIV, and self-regulating promoters (see newly issued US9624498);
- Nuclease Therapeutics: Treatments for HIV (see newly issued US9566352 and US 9757420), beta-thalassemia and SCD (see newly issued US9650698), hemophilia (see newly issued US9771403, US9777281 for hemophilia B) LSD (see newly issued US9877988), genome editing, Parkinson’s Disease, regulation of the expression of PD1; Immunomodulatory therapeutics; Cystic Fibrosis; CNS disease; Severe combined immunodeficiency (see newly issued US9161090 and US9833479, Modified T cells (See newly issued US9597357) including HLA knock out (see newly issued US9782437) and methods of editing stem cells (see newly issued US9834787);
- Non-Therapeutic Applications of ZFPs: Identification of genes, analysis of gene regulation, structure and biological function, methods of agricultural biotechnology, methods of altering cellular differentiation state;
- Non-Therapeutic Applications of ZFNs: Methods for identification of regulatory DNA sequences, prediction of patient response to drug therapeutics, and development of cell lines for improved protein production, methods of transgenic animal development (see newly issued UP9567573) , and methods of introducing exogenous nucleic acids of interest into a safe harbor locus, cleavage of specific miRNAs (see newly issued US9574211);
- Donor DNA design: Methods for designing optimal donors for transgene delivery;
- Pan-nuclease, Non-ZFP nucleases, methods of design and use (see newly issued US9765361), pan-nuclease nickases (see newly issued US9631186);
- Engineering of stem cells Methods of modulating stem cell differentiation (see newly issued US9624509); and
- Methods for genome editing.
Sangamo BioSciences has been advised that certain aspects of its technology can give it and its collaborators independence from third party patent claims to gene sequences. In general, under U.S. patent law, a patent may be obtained for any new and useful process, machine, manufacture, or composition of matter. An underlying theme of U.S. patent law, as related to biotechnology, is that the sequence of a gene, as it exists in the chromosome, is not patentable, even when newly discovered, although a cDNA corresponding to the transcription product of that gene may be in select instances. Accordingly, U.S. patent claims to DNA sequences can cover only isolated cDNAs, or modified nucleic acid sequences (e.g., a purified DNA fragment or a DNA sequence inserted into a vector). Sangamo BioSciences has been advised that U.S. patent claims to DNA sequences do not, and cannot, cover gene sequences as they exist in their natural chromosomal environment, and international patent law is even more stringent than U.S. patent law in this regard. Most current methods for over-expression of a gene or protein involve the introduction into a cell of a vector containing a DNA encoding the protein to be over-expressed. Because such a vector contains isolated cDNA sequences that encode the protein, it would be covered by any patent claims to those sequences. In contrast, its methods for over-expression utilize ZFP TFs that target endogenous genes as they exist in the chromosome. As a result, its gene regulation methods do not require the use of isolated cDNA sequences encoding the protein to be over-expressed and, its counsel has advised it, do not infringe patent claims to such sequences. Notwithstanding this advice, the company realize that others could take a contrary position that could result in litigation. While the company believe that the company would prevail in any such litigation, the uncertainties involved in litigation generally make it impossible to provide assurance as to the ultimate outcome of such matters. See “Risk Factors—Because it is difficult and costly to protect its proprietary rights, and third parties have filed patent applications that are similar to ours, the company cannot ensure the proprietary protection of its technologies and products.”
The patent positions of pharmaceutical and biotechnology firms, including its patent position, are uncertain and involve complex legal and factual questions for which important legal tenets are largely unresolved and are subject to interpretation and refinement by the court system. Patent applications may not result in the issuance of patents and the coverage claimed in a patent application may be significantly reduced before a patent is issued. Although Sangamo BioSciences has filed for patents on some aspects of its technology, the company cannot provide assurances that patents will be issued as a result of these pending applications or that any patent that has been or may be issued will be upheld. The laws of some foreign countries may not protect its proprietary rights to the same extent as do the laws of the United States. For example, its issued European patents EP2171052 and EP2527435 have been opposed in Europe. The company's EP2281050 case was revoked during Opposition in November 2016. Similarly, EP2171052 and EP2527435 underwent Opposition hearings in early 2017. Although these cases emerged from the Opposition hearings, the opponent filed appeals that are currently underway, and the company do not know what the outcome of these procedures will be. The claims of these patents may be amended such that claim scope is reduced or the patents may be revoked as a result of these procedures.
Manufacturing
The company rely on contract manufacturing organizations, or CMOs, to produce its preclinical and clinical product candidates in accordance with FDA and EMA mandated regulations, also known as current good manufacturing practices, or cGMPs. The company employ a technical operations staff in the areas of process development, analytical development, quality control, quality assurance, project management, and manufacturing to facilitate appropriate oversight of its CMOs, support of its regulatory filings and execution of clinical trials. In 2017, the company expanded its services agreement with Brammer Bio MA, LLC, or Brammer, to provide dedicated capacity to supply its preclinical and clinical programs. Additionally, the company plan to build a cGMP manufacturing facility at its new corporate headquarters in Brisbane, CA. This facility will be designed to manufacture of Phase 1/2 clinical trial supplies for its pipeline programs. The company believe this balanced approach to manufacturing, investing in internal capacity/capabilities while strengthening its commitment to external capacity, will enable it to meet its anticipated pipeline needs.
The company currently leverage two distinct manufacturing platforms: AAV vector production for its genome editing and gene therapy product candidates and HSPC modification for its cell therapy product candidates. The company use a commercial scale baculovirus manufacturing platform to manufacture AAV vectors for genome editing and gene therapy, with each AAV vector packaging a different transgene specific to the target indication or ZFN. The manufacturing process for its HSPC cell therapy product candidates utilizes the patient’s own HSPCs. These HSPCs are transfected using mRNA to produce ZFNs that target specific DNA sites, resulting in modified HSPCs.




