Synthetic Biologics
Synthetic Biologics (SYN) is a late-stage clinical stage company focused on developing therapeutics designed to preserve the microbiome to protect and restore the health of patients. Its lead candidates poised for Phase 3 development are: (1) SYN-004 (ribaxamase) which is designed to protect the gut microbiome from the effects of certain commonly used intravenous (IV) beta-lactam antibiotics for the prevention of C. difficile infection (CDI), overgrowth of pathogenic organisms and the emergence of antimicrobial resistance (AMR), and (2) SYN-010 which is intended to reduce the impact of methane-producing organisms in the gut microbiome to treat an underlying cause of irritable bowel syndrome with constipation (IBS-C). the companyare also developing preclinical stage monoclonal antibody therapies for the prevention and treatment of pertussis, and novel discovery stage biotherapeutics for the treatment of phenylketonuria (PKU).1
Product Pipeline:
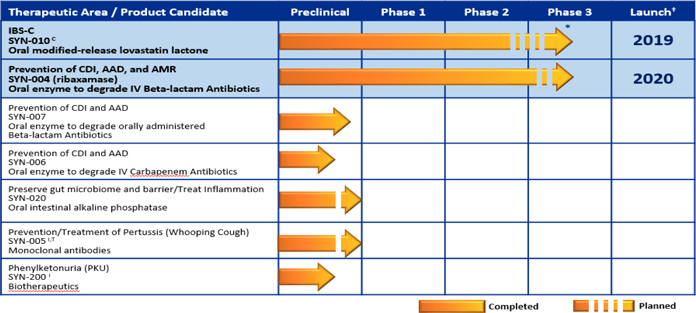
' * Two Phase 2 studies completed. Planning a Phase 2b/3 pivotal trial † Anticipated timing of launch contingent upon FDA approval C- Cedars-Sinai Medical Center Collaboration I- Intrexon Collaboration T- The University of Texas at Austin Collaboration
Clinical and Preclinical Programs
| Therapeutic Area | Product Candidate | Status | |
|---|---|---|---|
| Prevention of CDI, overgrowth of pathogenic organisms and AMR (Degrade IV beta-lactam antibiotics) | SYN-004 (ribaxamase) | · | Reported supportive Phase 1a/1b data (1Q 2015) |
| . | Initiated Phase 2b proof-of-concept clinical trial (3Q 2015) | ||
| . | Reported supportive topline data from first Phase 2a clinical trial (4Q 2015) | ||
| . | Reported supportive topline data from second Phase 2a clinical trial (2Q 2016) | ||
| . | Received USAN approval of the generic name “ribaxamase” for SYN -004 (July 2016) | ||
| . | Completed Enrollment of Phase 2b proof-of concept clinical trial (3Q 2016) | ||
| . | Awarded contract by the Centers for Disease Control and Prevention (CDC) (4Q 2016) | ||
| . | Announced positive topline data from Phase 2b proof-of-concept clinical trial, including achievement of primary endpoint of significantly reducing CDI (1Q 2017) | ||
| . | Announced additional results from Phase 2b proof-of-concept clinical trial demonstrating SYN-004 (ribaxamase) protected and maintained the naturally occurring composition of gut microbes from antibiotic-mediated dysbiosis in treated patients (Q2 2017) | ||
| . | Announced additional results from Phase 2b proof-of-concept clinical trial funded by a contract awarded by the CDC, demonstrating that SYN-004 (ribaxamase) prevented significant change to the presence of certain AMR genes in the gut resistome of patients receiving SYN-004 compared to placebo (Q3 2017) | ||
| . | Announced FDA granted Breakthrough Therapy Designation for the prevention of Clostridium difficile infection (CDI) (May 2017) | ||
| . | Submitted a request for a Type-B multidisciplinary meeting with the FDA to discuss the overarching, high-level drug development plan and pathway to marketing approval for SYN-004 (ribaxamase) (2H 2017) | ||
| . | Plan to initiate Phase 3 clinical trial(s) (1H 2018) | ||
| Treatment of IBS-C | SYN-010 (oral modified-release lovastatin lactone) | . | Reported supportive topline data from two Phase 2 clinical trials (4Q 2015 & 1Q 2016) |
| . | Received Type C meeting responses from U.S. Food and Drug Administration (FDA) regarding late-stage aspects of clinical pathway (2Q 2016) | ||
| . | Presented detailed data supporting previously reported positive topline data from two Phase 2 clinical trials at Digestive Disease Week Conference 2016 (DDW) (May 2016) | ||
| . | Held End of Phase 2 meeting with FDA (July 2016) | ||
| . | Confirmed key elements of Pivotal Phase 2b/3 clinical trial design pursuant to consultations with FDA (1Q 2017) | ||
| . | Collaboration with Cedars-Sinai Medical Center | ||
| Prevention of CDI, overgrowth of pathogenic organisms and AMR (Degrade oral beta-lactam antibiotics) | SYN-007 (oral enzyme) | . | Preclinical work ongoing to determine ability of SYN-007 to protect the gut microbiome and degrade oral beta-lactam antibiotics |
| Prevention and Treatment of pertussis | SYN-005 (monoclonal antibody therapies) | . | Reported supportive preclinical research findings (2014) |
| . | The University of Texas at Austin (“UT Austin”) received a grant from the Bill and Melinda Gates Foundation to support a preclinical study to evaluate the prophylactic capability of SYN-005 (4Q 2015) | ||
| . | Reported supportive preclinical data demonstrating SYN-005 provided protection from pertussis five weeks in neonatal non-human primate study (Q2 2017) | ||
| . | Collaborations with Intrexon and UT Austin |
Microbiome-Focused Pipeline
Synthetic Biologics IBS-C and CDI programs are focused on protecting the healthy function of the gut microbiome, or gut flora, which is home to billions of microbial species and composed of a natural balance of both “good” beneficial species and potentially “bad” pathogenic species. When the natural balance or normal function of these microbial species is disrupted, a person’s health can be compromised. All of the company programs are supported by its growing intellectual property portfolio. The company are maintaining and building its patent portfolio through: filing new patent applications; prosecuting existing applications; and licensing and acquiring new patents and patent applications. In total, The companyhold over 110 U.S. and foreign patents and have over 85 U.S. and foreign patents pending. Its plan remains focused on the advancement of its two late-stage clinical programs. The company continue its pursuit of successful and viable opportunities that will allow it to establish the clinical infrastructure and financial resources necessary to successfully initiate and complete this plan.
SYN-004 (ribaxamase) — Prevention of C. difficile infections (CDI) and antibiotic-associated diarrhea (AAD)
SYN-004 (ribaxamase) is an oral prophylactic therapy designed to degrade certain IV beta-lactam antibiotics within the gastrointestinal (GI) tract and maintain the natural balance of the gut microbiome for the prevention of CDI, overgrowth of pathogenic organisms and the emergence of antibiotic-resistant organisms. SYN-004 (ribaxamase) is a beta-lactamase enzyme which, when released in the proximal small intestine, can degrade beta-lactam antibiotics in the GI tract without altering systemic antibiotic levels. Beta-lactam antibiotics are a mainstay in hospital infection management and include the commonly used penicillin and cephalosporin classes of antibiotics.
In November 2012, The company acquired a series of oral beta-lactamase enzymes (P1A, P2A and P3A) and related assets targeting the prevention of CDI, the leading healthcare-associated infection that generally occurs secondary to treatment with IV antibiotics from Prev ABR LLC. The acquired assets include a pre-Investigation New Drug (IND) package for P3A, Phase 1 and Phase 2 clinical data for P1A, manufacturing processes and data, and a portfolio of issued and pending U.S. and foreign patents intended to support an IND and Biologics License Application (BLA) with the FDA. Utilizing this portfolio of assets, The company developed a proprietary, second generation oral beta-lactamase enzyme product candidate that The company now refer to as SYN-004 or by its generic name “ribaxamase”.
Compared to the first generation oral enzyme candidate of P1A, The company believe that the second generation candidate, SYN-004 (ribaxamase), will have activity against a broader spectrum of beta-lactam antibiotics, including both penicillins and certain cephalosporins. Due to the structural similarities between P1A and SYN-004 (ribaxamase), and based on previous discussions with the FDA, certain preclinical data collected on P1A were used in support of an IND application for its new product candidate, SYN-004 (ribaxamase).
Specifically, P1A had been evaluated in four Phase 1 and one Phase 2 clinical trials conducted in Europe. In total, 112 patients and 143 healthy normal subjects participated in these studies.
P1A (the first generation candidate) showed acceptable safety and tolerability in a Phase 1 clinical trial. In addition, data from two Phase 2 clinical trials demonstrated that P1A had the ability to preserve GI microflora in hospitalized patients treated with IV ampicillin or the combination of piperacillin and tazobactam.
In September 2016, The company completed enrollment in its randomized placebo-controlled Phase 2b proof-of-concept clinical trial intended to evaluate the ability of SYN-004 (ribaxamase) to prevent CDI, C. difficile associated diarrhea (CDAD) and AAD in patients hospitalized for a lower respiratory tract infection and receiving IV ceftriaxone.
On January 5, 2017, The company announced positive topline data from its Phase 2b clinical trial demonstrating SYN-004 (ribaxamase) achieved its primary endpoint of significantly reducing CDI. Preliminary analysis of the data indicated seven confirmed cases of CDI in the placebo group compared to two cases in the SYN-004 (ribaxamase) treatment group. Patients receiving SYN-004 (ribaxamase) achieved a 71.4% relative risk reduction (p-value=0.045) in CDI rates compared to patients receiving placebo. Adverse events reported during this trial were comparable between treatment and placebo arms. Results from this trial also demonstrated that patients administered ribaxamase in conjunction with IV-ceftriaxone demonstrated comparable cure rates (approximately 99%) for the treatment of primary infection compared to the placebo group.
Preliminary analysis of the data demonstrated a significant reduction in new colonization by vancomycin-resistant enterococci (VRE) for patients receiving SYN-004 (ribaxamase) compared to placebo (p-value=0.0002). With agreement from the FDA, the study included a secondary endpoint to assess SYN-004’s (ribaxamase) capacity to decrease the incidence of antibiotic-associated diarrhea from all causes. Preliminary analysis of the data suggested a trend towards such a reduction (p-value=0.13), which was due, for the most part, to the reduction of CDI.
On April 7, 2017, The company met with the CDC to share additional supportive results from several exploratory endpoints from its Phase 2b proof-of-concept clinical trial demonstrating SYN-004 (ribaxamase) successfully protected and preserved the naturally occurring composition of gut microbes in patients receiving SYN-004 (ribaxamase) from the dysbiotic effects of antibiotic-mediated intravenous ceftriaxone compared to placebo. Results indicate that patients who were administered SYN-004 (ribaxamase) in conjunction with IV ceftriaxone demonstrated significantly better maintenance of and recovery of the composition and diversity of the gut microbiome, compared to placebo. Patients receiving SYN-004 (ribaxamase) also demonstrated lower incidences of new colonization by opportunistic and potentially pathogenic microorganisms, such as VRE, compared to placebo.
The company is in the process of further analyzing data from this clinical trial and expect to share results from additional exploratory endpoints as they become available later this year, including results focused on the ability of SYN-004 (ribaxamase) to prevent the emergence of antimicrobial resistance in the gut microbiome.
On May 11, 2017, The company announced that the FDA granted a Breakthrough Therapy Designation (BTD) to SYN-004 (ribaxamase) for the prevention of Clostridium difficile infection (CDI). The Breakthrough Therapy Designation is based on data from the successful Phase 2b clinical trial of SYN-004 (ribaxamase), which met its primary endpoint of significantly reducing CDI. FDA Breakthrough Therapy Designation is intended to expedite development and review timelines when preliminary clinical evidence indicates that a drug may demonstrate substantial improvement on one or more clinically significant endpoints over available therapies for serious or life-threatening diseases. Following BTD, The company requested a Type-B multidisciplinary meeting with the FDA for a comprehensive discussion on the overarching, high-level drug development plan and pathway to marketing approval for SYN-004 (ribaxamase). If approved by the FDA, SYN-004 (ribaxamase) would be the first available drug designed to prevent Clostridium difficile infection by protecting the gut microbiome from antibiotic-mediated dysbiosis.
In 2017, The company also plan to continue collaborative efforts with CDC to gain public health support for SYN-004 (ribaxamase), hold an end of Phase 2 meeting with the FDA, and expect to initiate Phase 3 trial(s) towards the first half of 2018 or later, subject to its successful pursuit of opportunities that will allow it to establish the clinical infrastructure and financial resources necessary to successfully initiate and complete this plan.
Under a contract funded by the Centers for Disease Control and Prevention (CDC), The company have been examining the gut resistome (the content of the anti-microbial resistance genes of the gut microbiome) from the patients in its Phase 2b clinical study with ribaxamase. During this study, DNA extracted from 350 longitudinal fecal samples collected during the study were sequenced by whole genome shotgun sequencing. The DNA sequences were then interrogated against the Comprehensive Antimicrobial Resistant Database to determine the AMR genes present in the samples. A statistical analysis was then performed to compare the change in relative abundance of AMR genes of interest in the ribaxamase group vs. the placebo group. This analysis identified AMR genes that significantly changed from the screening sample to the post antibiotic samples. These changes included AMR genes that significantly increased and decreased following ceftriaxone treatment. There were approximately four-fold more genes that changed significantly in the placebo group as compared with the ribaxamase group. Among the genes that significantly increased in the placebo group are a family of five beta-lactamase genes which is consistent with the selective pressure from the ceftriaxone administered during the study. There were also several vancomycin resistance genes that increased in the placebo group which is consistent with the significant increase in colonization by vancomycin resistant enterococci seen in the placebo patients. The genes that decreased were mostly tetracycline and erythromycin resistance genes that are associated with normal gut flora. These data are consistent with ribaxamase degrading the ceftriaxone in the upper GI and thus relieving the selective pressure of the antibiotics on the gut microbiome.
SYN-010 — Treatment of Irritable Bowel Syndrome with Constipation (IBS-C)
SYN-010 is Synthetic Biologics proprietary, modified-release formulation of lovastatin lactone that is intended to reduce methane production by certain microorganisms (M. smithii ) in the gut while minimizing disruption to the microbiome. Methane produced by M. smithii is an underlying cause of pain, bloating and constipation associated with IBS-C, and published reports have associated higher intestinal methane production with increased constipation severity in IBS-C patients. SYN-010 is intended to act primarily in the intestinal lumen while avoiding systemic absorption, thereby targeting the major cause of IBS-C, not just the patient’s symptoms.
In December 2013, through its subsidiary Synthetic Biomics, Inc. (SYN Biomics), The company entered into a worldwide exclusive license agreement with Cedars-Sinai Medical Center (CSMC) and acquired the rights to develop products for therapeutic and prophylactic treatments of acute and chronic diseases, including the development of SYN-010 to target IBS-C. The company licensed from CSMC a portfolio of intellectual property comprised of several U.S. and foreign patents and pending patent applications for various fields of use, including IBS-C, obesity and diabetes. An investigational team led by Mark Pimentel, M.D. at CSMC discovered that these products may reduce the production of methane gas by certain GI microorganisms.
The company believe SYN-010 may reduce the impact of methane producing organisms on IBS-C.
Overview of two Phase 2 Clinical Trials
In 2015 and 2016, The company reported supportive data from its two SYN-010 Phase 2 trials, the first study was comprised of a randomized, double-blind, placebo-controlled, 4-week study comparing SYN-010 21 mg and 42 mg dose strengths to placebo (Study 1), followed by an open-label study in which eligible patients who completed Study 1 received SYN-010 42 mg for an additional 8 weeks (Study 2). The two Phase 2 SYN-010 clinical trials evaluated the change from baseline (Day 1 of Study 1) in breath methane, stool frequency and abdominal pain and bloating at the end of weeks 1, 4, 8 and 12 (Study 2 – Day 84) in patients diagnosed with IBS-C and with breath methane levels greater than 10 parts per million (ppm) at screening.
Allowance of Key U.S. Patent
On June 27, 2017, The company announced that the U.S. Patent and Trademark Office (USPTO) issued a Notice of Allowance for a patent which covers the use of the active agent of SYN-010, the Company’s proprietary, modified-release formulation of lovastatin lactone, for the treatment of constipation. Upon issuance, this patent will strengthen the intellectual property estate covering the use of SYN-010 for the treatment of IBS-C until at least 2034, affording the Company an extended term for commercialization.
Phase 3 Planning
On July 20, 2016, The company participated in an End of Phase 2 meeting with the FDA. Following a review of data from the two Phase 2 clinical trials of SYN-010 conducted by it , a collaborative and positive discussion ensued with the FDA to determine the optimal pathway to advance SYN-010 into Phase 3 development. On January 18, 2017, and in accordance with guidance from the FDA, The company confirmed its plan to conduct a Phase 2b/3 adaptive design study for its first pivotal trial intended to further evaluate the efficacy and safety of SYN-010. Which The company plan to initiate subject to its successful pursuit of opportunities that will allow it to establish the clinical infrastructure and financial resources necessary to successfully initiate and complete this plan.
In accordance with collaborative discussions with the FDA, key components of the SYN-010 Phase 2b/3 adaptive pivotal trial will include:
- A 12-week, multi-center, double-blind, placebo-controlled, adaptive design clinical trial;
- A study population of approximately 840 adult subjects diagnosed with IBS-C;
- Evaluation of efficacy and safety of two dose strengths of SYN-010 (21 mg and 42 mg) compared to placebo;
- Conducted in approximately 150 clinical sites in North America;
- Study subjects will be randomized in a 1:1:1 ratio, receiving either 21 mg of SYN-010, 42 mg of SYN-010, or placebo;
- Enrollment will be open to all IBS-C patients; breath-methane will be measured at baseline to ensure a comparable ratio of high-to-low breath methane IBS-C patients in each treatment arm; and
- An interim futility analysis may be conducted when approximately 50% of patients in each dosing arm have completed treatment.
Consistent with FDA written guidance, the primary objective for this study is to determine the efficacy of SYN-010, measured as an improvement from baseline in the percentage of overall weekly responders during the 12-week treatment period for SYN-010 21 mg and 42 mg daily doses compared to placebo. Secondary efficacy endpoints for both dose strengths of SYN-010 will measure changes from baseline in abdominal pain, bloating, bowel movement frequency and stool consistency. Exploratory outcomes include adequate relief and quality of life measures using the well-validated EQ-5D-5L and PAC-SYM patient questionnaires.
Anticipated Regulatory Strategy
Synthetic Biologics believe that it will be able to utilize the regulatory approval pathway provided in Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act (the “FDCA”) for SYN-010. A New Drug Application (NDA) submitted under Section 505(b)(2), referred to as a 505(b)(2) NDA, contains full safety and efficacy reports but allows at least some of the information required for NDA approval, such as safety and efficacy information on the active ingredient, to come from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The company believe it can rely in part on the FDA’s previous findings of safety for Mevacor (lovastatin) in published clinical data. The company expect to rely on published clinical trials using Mevacor to provide support of efficacy.
SYN-007 — Prevention of CDI and AAD
Preclinical work is ongoing to determine the ability of SYN-007 to degrade oral beta-lactam antibiotics and protect the gut microbiome. SYN-007 comprises a reformulated version of SYN-004 (ribaxamase) for use with oral beta-lactam antibiotics versus IV beta-lactam antibiotics.
SYN-006 — Prevention of CDI and AAD
The development of SYN-006 is in the discovery stage. SYN-006 is intended to be an oral prophylactic therapy designed to degrade IV carbapenem antibiotics (a third class of beta-lactam antibiotics) within the GI tract and maintain the natural balance of the gut microbiome for the prevention of CDI and AAD. While SYN-004 (ribaxamase) is intended to degrade penicillin and certain cephalosporins in the GI tract, the SYN-006 discovery program has the potential to expand the activity to a broader spectrum of IV beta-lactam antibiotics in the GI tract to include carbapenem antibiotics.
References
Infectious disease outbreaks are increasing while intervention options are declining due to widespread multidrug-resistant bacteria, increasing numbers of immuno-compromised patients (e.g., the elderly and cancer patients) and the isolation of new pathogens.
SYN-005 — Pertussis (Whooping Cough)
Intrexon Collaboration and The University of Texas (UT) at Austin Agreement
In August 2012, The company entered into a worldwide exclusive channel collaboration with Intrexon through which it intend to develop monoclonal antibody (mAb) therapies for the treatment of certain infectious diseases not adequately addressed by existing therapies. In December 2012, The company initiated mAb development for the prevention and treatment of pertussis focusing on toxin neutralization. Unlike antibiotics, The company are developing a mAb therapy to target and neutralize the pertussis toxin as a prophylaxis for high-risk newborns and in order to reduce the mortality rate in infected infants.
To further the development of this potential therapy for pertussis, The company entered into an agreement with UT Austin to license the rights to certain research and pending patents related to pertussis antibodies. These research efforts are being conducted at the Cockrell School of Engineering in the laboratory of Associate Professor, Jennifer A. Maynard, Ph.D., the Laurence E. McMakin, Jr. Centennial Faculty Fellow in the McKetta Department of Chemical Engineering. Dr. Maynard brings to the project her expertise in the development, optimization, and application of mAbs for the treatment of pertussis.
Synthetic Biologics previously reported that SYN-005, a cocktail of two mAbs, was highly efficacious as a therapeutic in non-human primates infected with B. pertussis. The data were published in Science Translational Medicine in December 2015.
In October 2015, the Bill & Melinda Gates Foundation awarded a grant to UT Austin to generate preclinical proof-of-concept data in the neonatal non-human primate model to test the hypothesis that antibody administration at birth may have a role in the prevention of pertussis.
In December 2015, the non-human primate prophylaxis study was initiated by UT Austin to determine if administration of hu1B7, one component of SYN-005, at two days of age could protect animals from a subsequent pertussis infection. On April 19, 2017, The company announced supportive preclinical data demonstrating hu1B7 provided five weeks of protection from pertussis in neonatal non-human primates. Control animals (n=6), infected with Bordetella pertussis (B. pertussis) at five weeks of age, demonstrated marked elevations in white blood cell counts and most exhibited behavioral signs of pertussis, including coughing and diminished activity. In contrast, the experimental animals (n=7), who were treated with hu1B7 at two days of age and then infected five weeks later, had significantly lower peak white blood cell counts (p=0.004) that remained within the normal range or were only slightly elevated. Importantly, all seven of the animals that received prophylactic hu1B7 appeared healthy and none exhibited any behavioral signs of pertussis. Building on this early success, The company have initiated preclinical testing of a modified version of hu1B7 that has the potential to extend the plasma half-life and substantially reduce the required dose of SYN-005.
This current study expands the potential clinical utility beyond therapy to also include prophylaxis.
SYN-200 — Treatment of Phenylketonuria (PKU)
In August 2015, The company initiated the SYN-200 discovery program for development and commercialization of novel biotherapeutics for the treatment of patients with PKU pursuant to an exclusive channel collaboration with Intrexon. The company are utilizing Intrexon’s ActoBiotics platform to provide a proprietary method of delivering therapeutic protein to the GI tract through food-grade microbes. This program is in the discovery stage.
SYN-020 — Oral Intestinal Alkaline Phosphatase
SYN-020 is in the preclinical development stage. SYN-020 is being developed as a modified-release oral dosage form of intestinal alkaline phosphatase (IAP). Published preclinical and clinical studies on IAP indicate that an oral IAP product may have efficacy in a broad range of significant therapeutic indications including inflammatory bowel disease, microbial dysbiosis and metabolic syndrome. The company has generated manufacturing cell lines and processes, and are initiating preclinical animal modeling for multiple novel indications.
Intellectual Property
All of Synthetic Biologics programs are supported by growing patent estates that The company either own or exclusively license. Each potential product has issued patents that provide protection. In total, The company has over 110 U.S. and foreign patents and over 85 U.S. and foreign patents pending. For instance, U.S. Patent Nos. 8,894,994 and 9,587,234, which include claims to compositions of matter and pharmaceutical compositions of beta-lactamases, including SYN-004 (ribaxamase), have patent terms to at least 2031. Further, U.S. Patent 9,301,995 and 9,301,996, both of which will expire in 2031, cover various uses of beta-lactamases, including SYN-004 (ribaxamase), in protecting the microbiome, and U.S. Patent Nos. 9,290,754, 9,376,673, 9,404,103, 9,464,280, and 9,695,409 which, will expire in at least 2035, covers further beta-lactamase compositions of matter related to SYN-004 (ribaxamase). Also, U.S. Patent No. 9,192,618, which expires in at least 2023, includes claims that cover use of statins, including SYN-010, for the treatment of IBS-C. U.S. Patent No. 9,289,418, which expires in at least 2033, includes claims that cover the use of a variety of compounds, including the active agent of SYN-010, to treat constipation in certain screened patients. Most recently, the USPTO granted a Notice of Allowance, of U.S. Patent Application No. 14/776,465 which, upon issuance, covers the method of use of the active agent of SYN-010 for the treatment of constipation until at least 2034. Pending applications US 14/826,115 and various foreign equivalent applications, cover SYN-010 formulations and, if issued, are expected to have a term to at least 2035.
Synthetic Biologics' goal is to ![]() obtain, maintain, and enforce patent protection for its products, formulations, processes, methods, and other proprietary technologies, (ii) preserve its trade secrets, and (iii) operate without infringing on the proprietary rights of other parties, worldwide. The company seek, where appropriate, the broadest intellectual property protection for product candidates, proprietary information, and proprietary technology through a combination of contractual arrangements and patents.
obtain, maintain, and enforce patent protection for its products, formulations, processes, methods, and other proprietary technologies, (ii) preserve its trade secrets, and (iii) operate without infringing on the proprietary rights of other parties, worldwide. The company seek, where appropriate, the broadest intellectual property protection for product candidates, proprietary information, and proprietary technology through a combination of contractual arrangements and patents.




