Vascular Biogenics
Overview
Vascular Biogenics (VBLT) is a clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of first-in-class treatments for cancer. The company conduct business under the name VBL Therapeutics. Vascular Biogenics was incorporated in Israel on January 27, 2000 as a company limited by shares under the name Medicard Ltd. In January 2003, the company changed its name to Vascular Biogenics Ltd. The company's program is based on its proprietary Vascular Targeting System, or VTS, platform technology, which utilizes genetically targeted therapy to destroy newly formed, or angiogenic, blood vessels, and which the company believe will allow it to develop product candidates for multiple oncology indications.1
The company's lead product candidate, VB-111 (ofranergene obadenovec), is a gene-based biologic that Vascular Biogenics is developing for solid tumor indications, and which Vascular Biogenics has advanced to programs for recurrent glioblastoma, or rGBM, an aggressive form of brain cancer, ovarian cancer and thyroid cancer. Vascular Biogenics has obtained fast track designation for VB-111 in the United States for prolongation of survival in patients with glioblastoma that has recurred following treatment with standard chemotherapy and radiation. Vascular Biogenics has also received orphan drug designation for GBM in both the United States and Europe. VB-111 has also received an orphan designation for the treatment of ovarian cancer by the European Medicines Agency. In September 2015, the company reported complete results from its Phase 2 trial of VB-111 in rGBM, demonstrating a statistically-significant benefit in overall survival and favorable response rate in patients treated with VB-111 in combination with bevacizumab. The company's pivotal Phase 3 GLOBE study in rGBM began in August 2015 and was comparing a combination of VB-111 and bevacizumab to bevacizumab alone. The study, which enrolled a total of 256 patients in the US, Canada and Israel was conducted under a special protocol assessment, or SPA, agreement with the U.S. Food and Drug Administration, or FDA, with full endorsement by the Canadian Brain Tumor Consortium (CBTC). On March 8, 2018, the company announced top-line results from the GLOBE study, which showed that the study did not meet its pre-specified primary endpoint of overall survival (OS). No new safety concerns associated with VB-111 have been identified in the GLOBE study. Once the company receive the full and final data set, the company will conduct an in-depth analysis in order to better understand the outcome of the GLOBE study and the potential activity of VB-111 in rGBM. The company do not think that results of the GLOBE study in rGBM will necessarily have implications on the prospects for VB-111 in other tumor types. The company's OVAL phase 3 potential registration study of VB-111 in platinum resistant ovarian cancer was launched in December 2017 and is conducted in collaboration with the GOG Foundation, Inc., a leading organization for research excellence in the field of gynecologic malignancies.
The company also have been conducting a program targeting anti-inflammatory diseases, based on the use of its Lecinoxoid platform technology. Lecinoxoids are a novel class of small molecules the company developed that are structurally and functionally similar to naturally occurring molecules known to modulate inflammation. The lead product candidate from this program, VB-201, is a Phase 2-ready molecule that demonstrated efficacy in reducing vascular inflammation in a Phase 2 sub-study in psoriatic patients with cardiovascular risk. Due to business limitations associated with the heavy burden of developing medications for cardiovascular diseases, the company chose to test it in psoriasis and ulcerative colitis; however, as the company reported in February 2015, VB-201 failed to meet the primary endpoint in Phase 2 clinical trials for psoriasis and for ulcerative colitis. As a result, Vascular Biogenics has terminated its development of VB-201 in those indications. Nevertheless, based on recent pre-clinical studies, the company believe that VB-201 and some second generation molecules such as VB-703 may be applicable for NASH and renal fibrosis, and the company may seek a clinical proof of concept in NASH patients through an exploratory Phase 2 study for VB-201. Since the company intends to focus its efforts and resources on advancing its oncology program, the company will seek to advance its Lecinoxoid assets via strategic deals.
Vascular Biogenics is also conducting a research program exploring the potential of targeting of MOSPD2 for immuno-oncology applications. In January 2017, the company reported that targeting of MOSPD2 inhibits chemotaxis of monocytes and neuropils, and that unpublished VBL data also show MOSPD2 expression on certain tumor cells. The company believe that targeting of MOSPD2 may have several therapeutic applications, including inhibition of monocyte migration in chronic inflammatory conditions, inhibition of tumor cell metastases and targeting of MOSPD2-expressing tumor cells. Vascular Biogenics is developing its “VB-600 series” of pipeline candidates towards these applications.
Vascular Biogenics is developing its lead oncology product candidate, VB-111, for solid tumor indications, with current clinical programs in rGBM, thyroid cancer and ovarian cancer. In interim analyses of data from its ongoing open-label Phase 2 clinical trial of VB-111 in rGBM, the company observed dose-dependent attenuation of tumor growth and an increase in median overall survival, which is the time interval from initiation of treatment to the patient’s death. The U.S. Food and Drug Administration, or FDA, has granted VB-111 fast track designation for prolongation of survival in patients with glioblastoma that has recurred following treatment with temozolomide, a chemotherapeutic agent commonly used to treat newly diagnosed glioblastoma, and radiation. On July 1, 2014, the FDA concurred with the design and planned analyses of its Phase 3 pivotal trial of VB-111 in rGBM pursuant to an SPA. At the time, commencement of the trial was subject to its providing the agency with more information regarding its potency release assay for the trial. The company developed this assay and submitted initial information to the FDA on May 26, 2014. On February 5, 2015 the FDA has found its data satisfactory and removed the partial hold.
The company began its Phase 3 pivotal trial of VB-111 in rGBM in August 2015 and completed patient enrollment for the study in December 2016, five months ahead of its initial plan. Following positive safety reviews announced in December 2016, in April 2017 and the third and final safety review that was announced in October 2017, the GLOBE trial continued to completion. On March 8, 2018, the company announced top-line results from the GLOBE study, which showed that the study did not meet its pre-specified primary endpoint of overall survival (OS).
VB-111 was also being studied in a Phase 2 trial for recurrent platinum-resistant Ovarian Cancer and in a Phase 2 study in recurrent, iodine-resistant differentiated Thyroid Cancer. In a Phase 2 trial for recurrent platinum-resistant ovarian cancer, VB-111 demonstrated a statistically significant increase in overall survival and 60% durable response rate (as measured by reduction in CA-125), approximately twice the historical response with bevacizumab plus chemotherapy in ovarian cancer. In December 2016, the company had an end-of-Phase-2 meeting with the FDA, in which the company received approval from the FDA to advance VB-111 for a Phase 3 study in platinum-resistant ovarian cancer, which the company launched in December 2017. The OVAL study is conducted in collaboration with the Gynecologic Oncology Group (GOG) Foundation, Inc., a leading organization for research excellence in the field of gynecologic malignancies.
In February 2017, the company reported full data from its exploratory Phase 2 study of VB-111 in recurrent, iodine-resistant differentiated thyroid cancer. The primary endpoint of the trial, defined as 6-month progression-free-survival (PFS-6) of 25%, was met with a dose response. Forty-seven percent of patients in the therapeutic-dose cohort reached PFS-6, versus 25% in the sub-therapeutic cohort, both groups meeting the primary endpoint. An overall survival benefit was seen, with a tail of more than 40% at 3.7 years for the therapeutic-dose cohort, similar to historical data for pazopanib (Votrient ®), a tyrosine kinase inhibitor; however, most patients in the VB-111 study had tumors that previously had progressed on pazopanib or other kinase inhibitors. As of December 31, 2016, the company had studied VB-111 in over 200 patients and have observed it to be well-tolerated. In December 2015, Vascular Biogenics has been granted a US composition of matter patents that provides intellectual property protection for VB-111 in the US until October 2033 before any patent term extension.
In June 2017, at the BIO international conference the company provided an update on the long-term status and survival of patients from three completed Phase 2 trials with VB-111. In the Phase 2 study in rGBM patients, 12-month survival was 54% in patients who were treated with VB-111 through progression, including a rGBM patient who remains alive with complete response after >50 months, compared to 23% of patients who had limited exposure of a therapeutic dose of VB-111. According to a meta-analysis, the 12-month survival on Avastin (bevacizumab) is only 24%. In the Phase 2 study in recurrent platinum-resistant and refractory ovarian cancer, 53% of patients treated with a therapeutic dose of VB-111 in combination with paclitaxel were alive at 15 months. No patients in the sub-therapeutic dose were alive at the 15-month time point. In the Phase 2 study in radioiodine refractory differentiated thyroid cancer, 53% of those who received multiple therapeutic doses of VB-111 were alive at 24 months, compared to 33% of those who received a single, sub-therapeutic dose of VB-111.
In October 2017, the company announced the opening of its new gene therapy manufacturing plant in Modiin, Israel. This plant can be the commercial facility for production of VB-111, if approved. The Modiin facility is the first commercial-scale gene therapy manufacturing facility in Israel and currently one of the largest gene-therapy designated ones in the world (20,000 sq. ft.). It is capable of manufacturing in large-scale capacity of 1,000 liters and is scalable to 2,000 liters.
In November 2017, the company signed an exclusive license agreement with NanoCarrier Co., Ltd. (TSE Mothers:4571) for the development, commercialization, and supply of VB-111 in Japan. VBL retains rights to VB-111 in the rest of the world. Under terms of the agreement, VBL has granted NanoCarrier an exclusive license to develop and commercialize VB-111 in Japan for all indications. VBL will supply NanoCarrier with VB-111, and NanoCarrier will be responsible for all regulatory and other clinical activities necessary for commercialization in Japan. In exchange, the company received an up-front payment of $15 million, and are entitled to receive greater than $100 million in development and commercial milestone payments. VBL will also receive tiered royalties on net sales in the high-teens.
Based on support from pre-clinical data, which the company presented at the American Society of Gene & Cell Therapy (ASGCT) conference in May 2017, the company planned to launch an exploratory study for VB-111 in combination with nivolumab, a checkpoint inhibitor, in non-small cell lung cancer. However, given the readout of the GLOBE trial, before the company launch such study, or studies, the company intend to conduct additional data analyses and revisit its clinical plans regarding new indications to seek the best way to advance VB-111 towards commercialization.
Strategy
The company's goal is to become a leading biopharmaceutical company focused on discovering, developing and commercializing innovative therapeutics that leverage its proprietary technologies for oncology indications. The company intend to achieve this goal by pursuing the following strategies:
- Pursue regulatory approval for its lead oncology compound, VB-111
The company believe VB-111 has the potential for applications in various solid tumors, and that the outcome of the GLOBE study in rGBM will not necessarily have implications on the prospects for VB-111 in other tumor types.
Vascular Biogenics has conducted Phase 2 clinical trials of VB-111 in both ovarian and thyroid cancer, with positive results. The company intend to continue development of VB-111 for platinum-resistant ovarian cancer, and launched a Phase 3 study for this indication in December 2017. Recently Vascular Biogenics has strengthened its balance sheet to support its development plans through a follow-on offering of $18 million on November 2017, and through a licensing deal for VB-111 in Japan, injecting a $15.0 million upfront payment in November 2017. The company may choose to advance VB-111 to additional cancer indications, either independently or through investigator-sponsored studies or strategic collaborations.
- Selectively enter into licensing and collaboration arrangements to supplement its internal development capabilities
As the company advance its pipeline of anti-cancer product candidates, the company will evaluate opportunities to selectively form collaborative alliances for its non-oncology assets to expand its capabilities and accelerate the development and commercialization of its oncology products. The company engage in conversations with third parties to evaluate such potential collaborations on an ongoing basis.
- Expand its manufacturing capacity to support clinical trials and possible commercialization of VB-111
The company previously manufactured clinical quantities of VB-111 at its facility in Or-Yehuda, Israel and through a third party in the United States. In October 2017, the company announced the opening of its new gene therapy manufacturing plant in Modiin, Israel. This plant can be the first commercial facility for production of VB-111 if it receives regulatory approval. On the longer term, the company intend to have more than one manufacturing site for VB-111, if regulatory approved.
Product Candidates and Technology
The following table summarizes the status of pipeline:
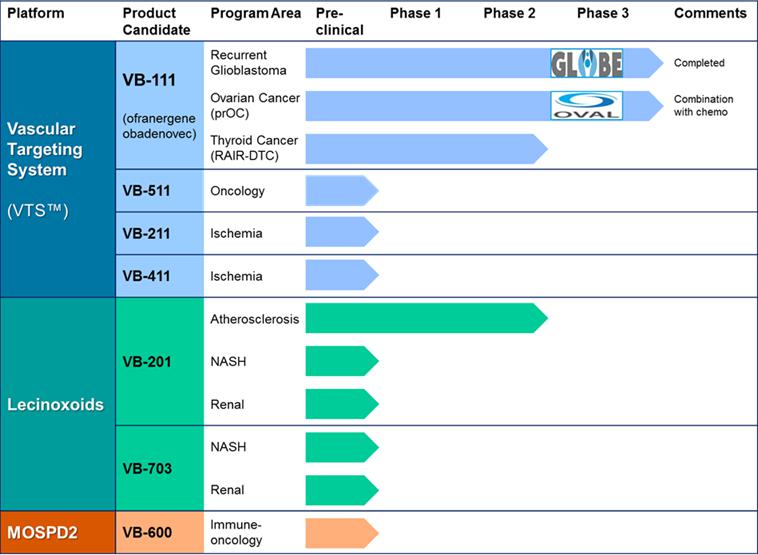
VTS Platform
Overview
The company's innovative, proprietary VTS platform technology enables systemic administration of gene therapy to either destroy or promote angiogenic blood vessels. VTS is both tissue- and condition-specific, allowing for targeted and limited gene expression in endothelial cells, the thin layer of cells that lines the interior surface of blood vessels undergoing angiogenesis.
The company's VTS platform technology comprises three components, a viral vector, a promoter and a transgene:
- Viral vector—a modified virus that is used as a delivery vehicle to distribute the promoter and the transgene throughout the body.
- Promoter—our proprietary, genetically modified promoter, called PPE-1-3X, that specifically targets the endothelial cells of angiogenic blood vessels. When present in these cells, the promoter initiates the expression of the transgene.
- Transgene—a genetic sequence designed to yield a specific biologic effect, the expression of which is directed by PPE-1-3X. The particular transgene will vary depending on the therapeutic objectives of the product candidate.
Once the gene therapy has reached the angiogenic blood vessels, the PPE-1-3X promoter activates expression of the transgene to produce a desired RNA or protein in the endothelial cells of those vessels. For oncology applications, the transgene selected is designed to destroy angiogenic blood vessels that feed solid tumors. For other potential applications, such as the treatment of ischemia, a different transgene can be selected that is designed to promote the development of angiogenic blood vessels instead of their destruction.
VB-111 (ofranergene obadenovec)
VB-111 is a unique biologic agent that uses a dual mechanism to target solid tumors. Its mechanism combines blockade of tumor vasculature with an anti-tumor immune response.
Based on a non-integrating, non-replicating, Adeno 5 vector, VB-111 utilizes VBL’s proprietary Vascular Targeting System (VTS™) to target the tumor vasculature for cancer therapy. The company designed VB-111 to address oncology indications, specifically solid tumors, by selectively targeting the blood vessels required for tumor growth and encouraging the programmed cell-death process, or apoptosis, of cells in those blood vessels. VB-111 is administered intravenously. PPE-1-3X is activated specifically in angiogenic endothelial cells and regulates a transgene consisting of a combination of two gene sequences known as Fas and TNFR1. When expressed, the transgene produces a unique pro-apoptotic protein, the Fas-TNFR1 chimera, that interacts with a native inflammatory molecule, Tumor Necrosis Factor, or TNF- alpha, and results in the destruction of newly formed or immature blood vessels. When activated by PPE-1-3X, specifically in angiogenic endothelial cells, this combination enables VB-111 to reduce tumor growth in a highly targeted manner.
In addition, VB-111 induces a specific anti-tumor immune response. In 2004, the company published preclinical data, which suggested that there is an immune inflammatory response to the presence of the viral vector and the Fas-TNFR1 chimera. Further support for a potential role of the immune system as part of VB-111’s mechanism of action came from an observation that patients who developed fever as a response to VB-111 administration, at least once along the treatment course, had a survival benefit over those who did not experience post-dosing fever. Moreover, an immunotherapeutic effect was also observed in biopsies taken from ovarian cancer patients. Immunohistochemistry staining showed regions of apoptotic cancer cells and infiltration of cytotoxic CD8 T-cells following treatment with VB-111.
In November 2017, at the Society for Neuro-Oncology conference, the company presented data, which support the relationship between VB-111’s novel dual immuno-oncology and vascular targeting mechanisms of action to overall survival, and show that molecular and radiographic biomarkers may serve as predictors of clinical benefit.
VB-111’s mechanism of action is illustrated below:
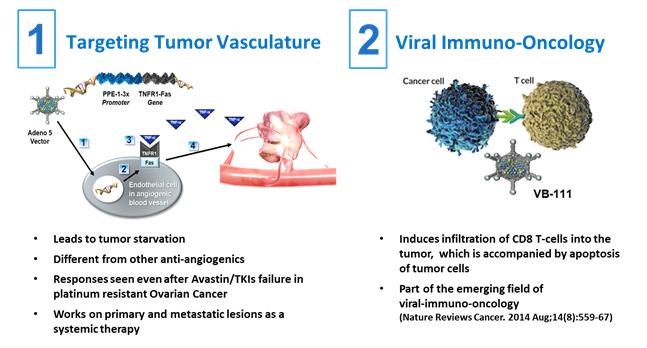
Unlike anti-VEGF agents (such as Avastin®) or tyrosine-kinase inhibitors (TKIs), VB-111 does not aim to block a specific pro-angiogenic pathway; instead, it uses an angiogenesis-specific sensor (VBL’s PPE-1-3x proprietary promoter) to specifically induce cell death in angiogenic endothelial cells in the tumor milieu. This mechanism may retain activity regardless of baseline tumor mutations or the identity of the pro-angiogenic factors secreted by the tumor and shows activity even after failure of prior treatment with other anti-angiogenics. Moreover, VB-111 induces specific anti-tumor immune response, which is accompanied by recruitment of CD8 T-cells and apoptosis of tumor cells. The company believe that this mode of action makes VB-111 less susceptible to resistance and, therefore, potentially applicable for a broader patient population than current therapies.
Vascular Biogenics has conducted pre-clinical studies in animal models of lung cancer, colon cancer, thyroid cancer, rGBM and melanoma. Based on those studies, and clinical results to date, the company believe that VB-111 has anti-tumoral activity that may hold clinical promise and may be suitable for treatment of some solid tumors. The company initially decided to focus on rGBM as its first indication because the company expected the rapid kinetics of this disease would enable it to accumulate clinical data in a short time, but the company also advanced VB-111 to a randomized-controlled Phase 3 study in platinum-resistant ovarian cancer.
VB-111 Clinical Programs- Overview
The company initially studied VB-111 in a Phase 1 “all comers” trial involving patients with multiple types of advanced metastatic cancer types, including thyroid cancer, neuroendocrine cancer, renal cell carcinoma and lung cancer. In that trial, VB-111 was well-tolerated and showed a dose- dependent extension in median overall survival across a range of tumor types. Based on these results, the company decided to proceed with the development of VB-111 for the lead indication of rGBM, as well as to investigate VB-111 as a monotherapy for the treatment of thyroid cancer and, in combination with chemotherapy, for ovarian cancer. Vascular Biogenics has an open IND for VB-111 with the Office of Cellular, Tissue, and Gene Therapeutics within FDA’s Center for Biologics Evaluation and Research.
VB-111 Clinical Program in GBM
Glioblastoma is a brain cancer that affects approximately 12,000 to 13,000 newly diagnosed people each year in the United States. It is a devastating, rapidly progressing tumor, with a median time from diagnosis to the patient’s death of 12 to 15 months. In recurrent glioblastoma, treatment consists of both symptomatic and palliative therapies. However, with currently available therapies, glioblastoma typically remains fatal within a very short period of time.
The company conducted an open-label Phase 2 trial in rGBM, which was originally initiated as an adaptive Phase 1/2 trial. The trial was intended to evaluate the safety and efficacy of VB-111, both by itself and in combination with bevacizumab, an anti-angiogenesis agent approved by the FDA for use in rGBM. In this trial, patients were initially dosed with VB-111 alone. After disease progression on VB-111 alone, they receive either bevacizumab alone as standard of care, or, in a second cohort, a combination of VB-111 and bevacizumab. Disease progression was defined as a worsening of the patient’s cancer with an increase of at least 25% in the overall mass of measurable tumors, the appearance of new tumors, the worsening of non-measurable tumors since beginning of treatment, a need for an increased dose of corticosteroids or clinical deterioration.
The company's Phase 2 trial results include 46 patients with rGBM treated with VB-111; upon disease progression, 23 patients were treated with VB-111 in combination with Avastin ®, and 22 received Avastin ® alone. One patient, who achieved a complete response, is still stable on VB-111 alone for more than 45 months as of December 31, and was included in the continuous exposure cohort. The median number of bi-monthly VB-111 doses was four for the cohort, which was treated with VB-111 through progression, versus one in the limited exposure cohort (average of 4.7 vs. 2.2, respectively). Continuous exposure to VB-111 demonstrated significant improvement in overall survival, with median overall survival of 59.1 weeks (414 days), compared to 31.9 weeks (223 days) in patients on limited VB-111 exposure (p=0.043), meeting the primary endpoint of the trial. Two complete responses and five partial responses were seen in the VB-111 continuous exposure cohort (n=24), compared to only two partial responses in VB-111 limited exposure cohort (n=22). VB-111 was found to be well tolerated.
Trial data also showed that VB-111 may induce an immuno-therapeutic effect. Of the 46 patients who received VB-111, 25 patients experienced a fever post-dosing of VB-111 at least once, while 21 patients did not. Patients with fevers demonstrated increased overall survival of 16 months, compared to patients without fevers, who had a median overall survival of 8.5 months (p=0.03). Additional biomarkers analyses presented at the SNO conference in November 2017 have demonstrated that in addition to fever, VB-111 is also associated with immune-mediated responses, including secretion of immune-stimulatory cytokines that correlate with OS, further supporting a role of the immune system as part of VB-111’s dual mechanism of action.
In June 2016 at the ASCO conference, the company presented clinical data that demonstrate a significant overall survival benefit in rGBM patients receiving VB-111 compared with historical Avastin ® meta-analysis data. In the Phase 2 VB-111 trial, the median overall survival of patients who received continuous exposure of VB-111 in combination with Avastin was 59.1 weeks. This is compared to 32.1 weeks in the pooled data from the 8 studies in the meta-analysis (p=0.0295; Hazard Ratio 0.62, 95% CI: 0.40-0.96). Median survival ranged from 26.0 weeks to 45.7 weeks in the meta-analysis. Overall survival at 12 months for patients on continuous exposure of VB-111 was 57%, compared with 24% overall survival (range 16%-38%) for the pooled Avastin ® treated rGBM data (p=0.03).
In 62 patients with rGBM, the most frequent toxicity was self-limited fever, starting several hours post therapy and usually resolving within 24 hours and controlled with anti-pyretics. There were 22 adverse events classified as grade 3 or higher, of which 7 were considered possibly related to VB-111 including asthenia, pyrexia, brain edema, depressed consciousness, pulmonary embolism, or PE (in a patient with PE prior to enrollment in the trial) and hypertension. Safety results were reviewed five times by the trial Data and Safety Monitoring Board, as well as by the FDA, without safety concerns. Based on interim Phase 2 data of VB-111 in rGBM, the FDA has allowed VBL to launch a Phase 3 study of VB-111 in rGBM patients even prior to the completion of the Phase 2 trial.
In June 2016 at the ASCO conference, the company presented clinical data that demonstrate a significant overall survival benefit in rGBM patients receiving VB-111 compared with historical Avastin ® meta-analysis data. In the Phase 2 VB-111 trial, the median overall survival of patients who received continuous exposure of VB-111 in combination with Avastin was 59.1 weeks. This is compared to 32.1 weeks in the pooled data from the 8 studies in the meta-analysis (p=0.0295; Hazard Ratio 0.62, 95% CI: 0.40-0.96). Median survival ranged from 26.0 weeks to 45.7 weeks in the meta-analysis. Overall survival at 12 months for patients on continuous exposure of VB-111 was 57%, compared with 24% overall survival (range 16%-38%) for the pooled Avastin ® treated rGBM data (p=0.03).
VBL’s pivotal Phase 3 GLOBE study was conducted under a Special Protocol Assessment (SPA) granted by the FDA, with full endorsement by the Canadian Brain Tumor Consortium (CBTC). Enrollment in the study, 256 patients in total, started in August 2015 and has been completed in December 2016, five months ahead of schedule.
To maintain an SPA agreement, FDA approval is required for any protocol modification. Following a meeting with FDA in December 2016, VBL has received FDA approval for adjustments in the GLOBE protocol, while keeping the SPA in place. Originally, the interim DSMC analysis in GLOBE was to be conducted after 91 deaths. The modified protocol specified that it would be conducted after 105 deaths, and after 50% of the patients have more than 12 months potential follow up, whichever occurs later. The final analysis would be conducted at 189 deaths (75% of events), versus the original planned for 151 deaths (60% of events). In late 2017, FDA had also approved the use of 12-month survival rate and stratification according to baseline tumor volume as study endpoints under the SPA, along with an exploratory analysis of survival starting at 100 days.
Three safety reviews were conducted during the GLOBE trial, by the independent Data Safety Monitoring Committee (DSMC). The DSMC is an independent multidisciplinary group that conducts detailed review of un-blinded study data, discusses potential safety concerns and provides recommendations regarding trial continuation. In December 2016, the company announced that the independent Data Safety Monitoring Committee (DSMC) met to conduct its first safety review of the Phase 3 GLOBE Study investigating ofranergene obadenovec (VB-111) in recurrent glioblastoma (rGBM). The committee reviewed the GLOBE safety data collected through a cutoff date in September 2016, and did not find any adverse events that would be cause for concern. As a result, the DSMC recommended that the study continue as planned. In April 2017, the company announce that the committee reviewed the GLOBE safety data collected through a cutoff date in March 2017 and unanimously recommended that the study continue as planned. The Third and final DSMC review took place in September 2017. The committee reviewed the GLOBE safety data, including mortality data, collected through a cutoff date in August 2017 and stated that they did not identify any safety concerns. The DSMC confirmed that no additional follow up will be necessary. Accordingly, the DSMC unanimously recommended that the study continue as planned, to completion.
On March 8, 2018 the company announced top-line data from the GLOBE study. These results show that the GLOBE study did not meet its pre-specified primary endpoint of overall survival (OS), or the secondary endpoint of progression-free-survival (PFS). No new safety concerns associated with VB-111 have been identified in the GLOBE study. Once the company receive the full and final data set, the company intend to conduct an in-depth analysis in order to better understand the outcome of the study and the potential activity of VB-111 in rGBM.
VB-111 Clinical Program in Ovarian Cancer
In addition to GBM, based on observations from early clinical trials, Vascular Biogenics has advanced VB-111 into tumor specific, repeat-dose trials, in ovarian cancer and thyroid cancer.
Ovarian cancer was diagnosed in approximately 22,000 American women in 2013, according to the National Cancer Institute. In ovarian cancer, clinical trials of bevacizumab, which, like VB-111, is an anti-angiogenic agent, demonstrated some improvement in progression free survival in women with high-risk advanced ovarian cancer. Therefore, the company conducted a Phase 1/2 clinical trial in ovarian cancer using VB-111 in combination with paclitaxel, a common chemotherapeutic agent.
This trial was designed as a Phase 1/2 dose escalation study. The primary objectives were to evaluate the safety and tolerability and identify dose limiting toxicity in combination of VB-111 and weekly paclitaxel; and explore the efficacy in an expanded cohort of the optimally tolerated dose of combination VB-111 and weekly paclitaxel, based on RECIST response, CA-125 response, progression free survival (PFS) and overall survival (OS) in patients with recurrent platinum-resistant ovarian cancer.
Twenty one patients with recurrent platinum-resistant Müllerian/ovarian cancer were enrolled at Massachusetts General Hospital and Dana Farber Cancer Institute, and received up to 7 doses of treatment. Patients were treated in two consecutive cohorts: Low Dose Treatment (n=4, 3x10 12 VPs + 40mg/80 mg paclitaxel) or a Therapeutic Dose (n=17, 1x10 13 VPs + 80 mg paclitaxel). All patients had measurable disease, with a grade at diagnosis of: 1A (1, 5%), 1B (1, 5%), 1C (1, 5%); IIIC (12, 57%); or IV (6, 29%). The patients included in the study were of particularly adverse prognosis as 48% of the patients were primary platinum refractory and 52% had tumors that failed to respond to prior anti-angiogenic agents, including Avastin.
In June 2016 at the ASCO conference, the company presented clinical top-line data from this trial. The results showed a significant increase in overall survival at the therapeutic dose of VB-111 vs. the low dose level (810 vs. 172 days, p=0.042). Nine of the 15 evaluable patients (60%) on the therapeutic dose had a response, as defined by a 50% reduction in CA-125. Durable RECIST responses and disease stabilizations were seen. This represents an approximate doubling in response rate, compared to historical data with ovarian cancer patients treated with a combination of Avastin ® and chemotherapy in the AURELIA trial which reported CA-125 response in 11.6% of patients treated with chemotherapy and 31.8% CA-125 response in ovarian cancer patients treated with a combination of chemotherapy and Avastin.
An immunotherapeutic effect was also observed in biopsies taken from patients. H&E and immunohistochemistry staining showed regions of apoptotic cancer cells and infiltration of cytotoxic CD8 T-cells following treatment with VB-111. VB-111 was found to be safe and well tolerated. Toxicity was similar to what would be expected with antiangiogenics and taxanes in this patient population. Eight serious adverse events were reported, 2 were considered by the investigator to be possibly related to be VB-111. No dose limiting toxicities were reported at any dose level.
In December 2016 the company had an end-of-Phase 2 meeting with the FDA to discuss the clinical path of VB-111 in ovarian cancer. The company reached agreement with the FDA on its clinical plan to proceed to a Phase 3 potential registration study of VB-111 in platinum-resistant patients, with OS as the primary endpoint. The company intend to advance VB-111 for this patient population, for which most current therapies fail to prolong patient survival, and in December 2017 announce the enrollment of the first patient in the OVAL potential registration Phase 3 study of VB-111 in platinum-resistant ovarian cancer. The OVAL study will be conducted in collaboration with the Gynecologic Oncology Group (GOG) Foundation, Inc., a leading organization for research excellence in the field of gynecologic malignancies.
The randomized, controlled, double-blind, Phase 3 OVAL study in recurrent platinum-resistant ovarian cancer has been designed to enroll up to 350 adult patients at approximately 75 clinical sites in the United States and Israel. Patients will be randomized 1:1 to VB-111 (1x10 13 VPs once every 8 weeks) in combination with chemotherapy (80mg/m2 paclitaxel once weekly), or to placebo with chemotherapy. The primary endpoint is overall survival. Additional endpoints include objective response rate (ORR), progression free survival (PFS), CA-125, RECIST 1.1 response and patient reported outcome measures. Given the GLOBE trial results, the company may choose to adapt the OVAL trial protocol to provide better insight and support of activity of VB-111 in this indication during the study.
VB-111 Program in Thyroid Cancer
The company conducted an exploratory Phase 2 clinical trial to evaluate safety and efficacy of VB-111 in advanced thyroid cancer. According to the National Cancer Institute, there are an estimated 535,000 people currently living with thyroid cancer in the United States, with an estimated 60,000 new cases of thyroid cancer each year. Most cases can be treated by surgery and radioactive iodine. If radioactive iodine is ineffective, other treatments are prescribed, such tyrosine kinase inhibitors and systemic chemotherapy. However, if such treatments are unsuccessful, the therapeutic options for patients are currently very limited. There are an estimated 2,000 annual deaths in the U.S. as a result of the disease. This subset of patients has an unmet need for novel therapeutic options such as VB-111.
The company's open-label dose-escalating study enrolled patients with advanced, recently-progressive, differentiated thyroid cancer that was unresponsive to radioactive iodine, in two cohorts. Most patients had tumors that had not responded to multiple therapies prior to enrollment, including radiation and kinase inhibitors. In the first cohort, thirteen patients received a single intravenous infusion of VB-111 at a sub-therapeutic dose of 3X10 12 viral particles (VPs). The second cohort included seventeen patients, who received VB-111 at a therapeutic dose of 10 13 VPs every two months until disease progression. One patient proceeded from a single low dose to receive later multiple high doses at progression and was included in both groups (for PFS only).
On February 21, 2017 the company announced full data from this trial. The primary endpoint of the trial, defined as 6-month progression-free-survival (PFS-6) of 25%, was met with a dose response. Forty-seven percent (47%; 8/17) of patients in the therapeutic-dose cohort reached PFS-6, versus 25% (4/12) in the sub-therapeutic cohort, both groups meeting the primary endpoint. Reduction in tumor measurement after the first dose was seen in 44% (7/16) of patients in the therapeutic-dose cohort, compared to 9% (1/11) in the sub-therapeutic-dose cohort. An overall survival benefit was seen with a tail of more than 40% at 3.7 years for the therapeutic-dose cohort (mOS 684 days). This is similar to historical data for pazopanib (Votrient ® ), a tyrosine kinase inhibitor; however, most patients in the VB-111 study had tumors that previously had progressed on pazopanib or other kinase inhibitors. VB-111 was observed to be well-tolerated in this study, with no signs of clinically significant safety issues.
The company see these positive data as additional proof-of-concept for VB-111 in another advanced solid tumor, particularly important for investigating the therapeutic potential of VB-111 even as monotherapy. The company's primary focus continues to be advancement of VB-111 towards commercialization, if approved, in ovarian cancer. Further clinical development of VB-111 for thyroid cancer may also be pursued, potentially with a strategic partner, or via an investigator-sponsored study.
** Additional VB-111 Program**
Based on support from pre-clinical data, which the company presented at the American Society of Gene & Cell Therapy (ASGCT) conference in May 2017, the company planned to conduct an exploratory study for VB-111 in combination with nivolumab, a checkpoint inhibitor, in non-small cell lung cancer. Launch of this trial was expected in the first quarter of 2018. However, given the readout of the GLOBE trial, before the company launch such study, or studies, the company intend to conduct additional data analyses and revisit its clinical plans regarding new indications, to seek the best way to advance VB-111 towards commercialization.
Additional VTS Pipeline candidates
The company's VTS platform technology enables systemic administration of gene therapy to either destroy or promote angiogenic blood vessels. Beyond VB-111, Vascular Biogenics has generated additional product candidates which utilize the same vector and promoter as in VB-111, yet comprise alternative functional transgenes. VB-511 is an anti-angiogenic candidate, while VB-211 and VB-411 are pro-angiogenic candidates that may be employed for ischemic conditions like peripheral vascular disease. All three candidates have demonstrated pre-clinical efficacy and are ready for toxicology. The company may pursue further development of VB-511 internally, but the company aim to partner-out the pro-angiogenic candidates.
Lecinoxoid Platform Technology
The company's proprietary Lecinoxoid platform technology comprises a family of orally administered small molecules designed to modulate the body’s inflammatory response. Lecinoxoids are compounds that are structurally and functionally similar to naturally occurring molecules, known as oxidized phospholipids, which possess immune modulating anti-inflammatory properties, modified to enhance stability and activity. The company believe that Lecinoxoids hold significant promise in their ability to treat a range of chronic immune-based inflammatory diseases.
The inflammatory response is a complex physiologic process balancing both pro- and anti- inflammatory components that interact intimately with the body’s immune system. Oxidized phospholipids are instrumental in the interplay of these components that maintain equilibrium. When the inflammatory response is not adequately balanced, excess inflammation results and may cause both acute and chronic disease states.
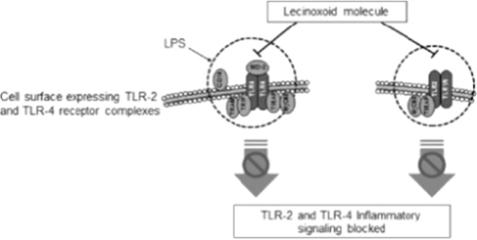
The Lecinoxoid platform seeks to harness the ability of oxidized phospholipids to regulate and attenuate key immune-inflammatory signaling. The company believe that its approach—identifying naturally occurring anti-inflammatory compounds and modifying them to enhance stability and activity—may lead to more physiologically balanced responses than other available anti-inflammatory therapies.
VB-201
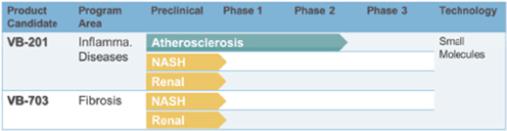
The company's lead Lecinoxoid-based compound, VB-201, was designed as an oral agent for the control of chronic inflammatory disorders. It was clinically developed for psoriasis and ulcerative colitis, however, its recent Phase 2 data do not support further development for these indications. The company believe that VB-201 may still have potential in other disorders in which TLR-2 and TLR-4 or monocytes play a role. In this regard, in April 2016 the company announced a scientific publication of preclinical findings demonstrating that VB-201, and its next-generation derivative VB-703, can inhibit Non-Alcoholic Steatohepatitis, or NASH, and liver fibrosis in a murine NASH model.
Non-alcoholic fatty liver disease is the most common liver disease in the western world. This pathological condition is characterized by lipid accumulation in hepatocytes and ranges from the non-progressive form termed steatosis to NASH, the progressive form that is prone to the development of cirrhosis, liver cancer, and liver failure. NASH is characterized by fatty liver inflammation. Several studies have implicated TLR4 and its co-receptor CD14 in NASH and fibrosis. In addition, studies have emphasized the crucial role of infiltrating monocytes/macrophages for the progression of liver inflammation and fibrosis in experimental mouse models and in patients with liver cirrhosis. It has become clear that the macrophage compartment of the liver, traditionally called Kupffer cells, is greatly augmented by an overwhelming number of infiltrating monocytes upon acute or chronic liver injury.
VB-201 inhibits the CD-14/TLR4 and TLR2 pathways as well as monocyte migration. Using an external CRO, the company studied VB-201 in a mouse model of NASH and found that while treatment with VB-201 did not reduce steatosis, it significantly decreased liver lobular inflammation and liver fibrosis compared to vehicle treated mice. Furthermore, in April 2017 VBL presented findings of a post-hoc, hypothesis-driven analysis of data from completed Phase 2 studies with VB-201, indicating that VB-201 may reduce certain liver enzymes in blood samples from treated patients, in a time-dependent and dose-dependent manner. VBL may seek to strengthen these findings and investigate the potential of VB-201 through an exploratory Phase 2 study in NASH patients.
Beyond VB-201, Vascular Biogenics has developed second and third generations of Lecinoxoid product candidates. The company's results highlight the potential of some of these molecules, such as VB-703, a third generation candidate whose IP life-cycle can extend to the mid-2030s. In May 2015 at the DDW conference, the company presented that VB-703 inhibits liver fibrosis by blocking TLR4 signaling pathways.
Recent preclinical studies which the company performed also demonstrate efficacy of VB-201 and VB-703 in a rat model of renal fibrosis. Renal fibrosis is a direct consequence of the kidney’s limited capacity to regenerate after injury. Renal scarring results in a progressive loss of renal function, ultimately leading to end-stage renal failure and a requirement for dialysis or kidney transplantation.
The company's lead Lecinoxoid molecule, VB-201, is a Phase-2 ready oral molecule which has demonstrated safety in > 600 patients and proof-of-concept in a Phase 2 study for vascular inflammation. The company's next-generation molecule VB-703, offers long IP lifecycle with demonstrated efficacy in liver and renal fibrosis models.
The following table summarizes the status of the Lecinoxoid platform:
The company believe that Lecinoxoids have therapeutic potential in disorders in which TLR-2 and TLR-4 or monocytes play a role, such as atherosclerosis, NASH/Liver fibrosis and renal fibrosis. In spite of that potential, Vascular Biogenics has decided strategically to focus its efforts and resources on development of novel anti-cancer therapies, such as VB-111 and MOSPD2. Therefore, the company may seek some proof-of-concept findings for Lecinoxoids , but on the longer term the company intend to seek one or more strategic partners that would help it to promote the development of its Lecinoxoid assets.
Expanding its Pipeline—The VB-600 Family
The company intend to continue research and development activities in the oncology field to strengthen the pipeline of its anti-cancer drug candidates. In this regard, the company believe that Vascular Biogenics has identified a novel tumor-related target, MOSPD2, that may be used as a marker for selective targeting of several types of tumors. In January 2017, a manuscript published by VBL disclosed that MOSPD2, a protein with a previously unknown function, regulates cell migration in human monocytes. While this first manuscript focused on the importance of MOSPD2 in immune cells, research conducted by VBL has explored the relevance of MOSPD2 in motility and metastasis of tumor cells. These oncology-related data were presented at the American Association of Cancer research (AACR) conference in April 2017. The company believe that targeting of MOSPD2 may have several therapeutic applications, including inhibition of monocyte migration in chronic inflammatory conditions, inhibition of tumor cell metastases and targeting of MOSPD2-expressing tumor cells. Vascular Biogenics is developing its VB-600 series of pipeline candidates towards these applications. The company expect to present additional findings related to its MOSPD2 program for cancer in the second quarter of 2018.
Intellectual Property
The company's success depends, at least in part, on its ability to protect its proprietary technology and intellectual property, and to operate without infringing or violating the proprietary rights of others. The company rely on a combination of patent, trademark, trade secret and copyright laws, know- how, intellectual property licenses and other contractual rights, including confidentiality and invention assignment agreements to protect its intellectual property rights.
Patents
As of January 28, 2018, the company had 178 granted patents and 39 applications pending worldwide for its oncology program and VTS platform technology and 110 granted patents and 30 patent applications pending worldwide for its anti-inflammatory program and Lecinoxoid family of compounds. The company's lead VTS asset, VB-111, is covered by US granted patent extending to 2033 before any extensions. The company's lead Lecinoxoid, VB-201, is protected by US granted composition-of-matter patent extending to 2027 before any extensions. In addition, Vascular Biogenics has pending patent applications covering use of VB-201, VB-703 and additional Lecinoxoid for NASH and fibrosis indications that may extend, if granted, to the 2030s. For MOSPD2, there are 2 applications pending worldwide.
Trademarks
The company rely on trade names, trademarks and service marks to protect its name brands. The company's registered trademarks in several countries include the following: “VTS,” “VASCULAR TARGETING SYSTEMS,” “VBL,” “V VBL THERAPEUTICS & Design,” “VASCULAR BIOGENICS,” “VASCULAR THERAPEUTICS” and “GLOBE & Design.”
Trade Secrets and Confidential Information
In addition to patented technology, the company rely on its unpatented proprietary technology, trade secrets, processes and know-how. The company rely on, among other things, confidentiality and invention assignment agreements to protect its proprietary know-how and other intellectual property that may not be patentable, or that the company believe is best protected by means that do not require public disclosure. For example, the company require its employees to execute confidentiality agreements in connection with their employment relationships with it, and to disclose and assign to it inventions conceived in connection with their services to it. However, there can be no assurance that these agreements will be enforceable or that they will provide it with adequate protection.
The company may be unable to obtain, maintain and protect the intellectual property rights necessary to conduct its business, and may be subject to claims that the company infringe or otherwise violate the intellectual property rights of others, which could materially harm its business. For a more comprehensive summary of the risks related to its intellectual property, see “Risk Factors.”
Sales and Marketing
Vascular Biogenics has not yet established sales, marketing or product distribution operations because its lead candidates are still in early clinical development.
Manufacturing
The company generally perform process development for its drug substance candidates and manufacture quantities of its drug candidates necessary to conduct pre-clinical studies and clinical trials of its drug candidates. The company rely on third-party manufacturers to produce bulk drug substance required for its clinical trials and expect to continue to rely on third parties to manufacture clinical trial drug supplies for the foreseeable future. The company also contract with additional third parties for the formulating, labeling, packaging, storage and distribution of the final drug products.
VB-111
Until late 2017, the company manufactured the active pharmaceutical ingredient and the formulated drug product of VB-111 for the clinical development at its small-scale cGMP-compliant production facility in Or-Yehuda, Israel and pursuant to an arrangement with a third party in the United States.
In October 2017, the company announced the opening of its new gene therapy manufacturing plant in Modiin, Israel. This plant will be the commercial facility for production of the Company’s lead product candidate, ofranergene obadenovec (VB-111), if approved. The site design enables modular expansion of the manufacturing capacity, to supply growing demand following commercialization. The Modiin facility shall also enable it to comply with the restrictions of the Research Law and its undertaking to the OCS that an essential portion of its VB-111 production, and in any event not less than the majority of VB-111 production, will remain in Israel. The investment in the facility is included in the Company’s budget and was also supported by the Israel Innovation Authority. VBL expects that its current cash will fund the Company’s operating expenses and capital expenditure requirements through 2020.
Employees
As of March 1, 2018, the company employed 37 employees, including 30 in research and development, and 7 in general and administrative positions, and of which 14 employees have either MDs or PhDs. All of its employees are located in Israel. The company believe its employee relations are good.
Israeli labor laws govern the length of the workday, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination of employment, equal opportunity and anti- discrimination laws and other conditions of employment. Subject to specified exceptions, Israeli law generally requires severance pay upon the retirement, death or dismissal of an employee, and requires it and its employees to make payments to the National Insurance Institute, which is similar to the U.S. Social Security Administration. The company's employees have defined benefit pension plans that comply with the applicable Israeli legal requirements.
None of its employees currently work under any collective bargaining agreements.
Property
The company's corporate headquarters and research facilities are currently located in Modi’in, Israel, where the company lease an aggregate of approximately 21,500 square feet of office and laboratory space, pursuant to a lease agreement that expires in June 2023. This facility additionally houses its clinical development, clinical operations, regulatory and management functions, as well as its local biological drugs manufacturing facility.




