AEterna Zentaris
History and development of the Company
AEterna Zentaris (AEZS) is a specialty biopharmaceutical company engaged in developing and commercializing pharmaceutical therapies, currently focused on the development and commercialization of Macrilen™ (macimorelin), including through out-licensing arrangements and pursuing in-licensing opportunities.
AEterna Zentaris was incorporated on September 12, 1990 under the Canada Business Corporations Act (the "CBCA") and continue to be governed by the CBCA. The company's registered address is located at 1155 René-Lévesque Blvd, West 41st Floor, Montréal, Quebec, Canada H3B 3V2 c/o Stikeman Elliott, LLP. The company's executive offices are located at 315 Sigma Drive, Summerville, South Carolina 29486; its telephone number is (843) 900-3223 and its website is <<<www.aezsinc.com>>>. None of the documents or information found on its website shall be deemed to be included in or incorporated by reference into this Annual Report on Form 20-F, unless such document is specifically incorporated herein by reference.
On December 30, 2002, the company acquired Zentaris AG, a biopharmaceutical company based in Frankfurt, Germany. Zentaris was a spin-off of Asta Medica GmbH, a former pharmaceutical company affiliated with Degussa AG.
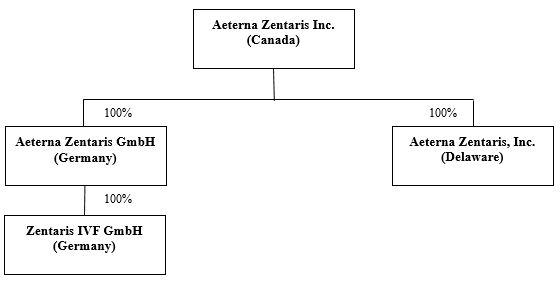
In May 2004, the company changed its name to Aeterna Zentaris Inc. and on May 11, 2007, Zentaris GmbH was renamed Aeterna Zentaris GmbH ("AEZS Germany"). AEZS Germany conducts its drug development efforts. In September 2007, the company incorporated Aeterna Zentaris, Inc. under the laws of Delaware. This wholly-owned subsidiary, which is based in the Charleston, South Carolina area, conducts certain of its administrative and commercial operations.
On November 17, 2015, the company effected a 100-to-1 Share Consolidation (reverse stock split). The company's Common Shares commenced trading on a consolidated and adjusted basis on both NASDAQ and TSX on November 20, 2015.
The company currently have three wholly-owned direct and indirect subsidiaries, AEZS Germany, based in Frankfurt, Germany; Zentaris IVF GmbH, a direct wholly-owned subsidiary of AEZS Germany based in Frankfurt, Germany; and Aeterna Zentaris, Inc., an entity incorporated in the State of Delaware with an office in the Charleston, South Carolina area in the United States.
The company's Common Shares are listed for trading on both NASDAQ and TSX under the trading symbol "AEZS".
The company's agent for service of process and SEC matters in the United States is its wholly-owned subsidiary, Aeterna Zentaris, Inc., located at 315 Sigma Drive, Summerville, South Carolina 29486.
There have been no public takeover offers by third parties with respect to it or by it in respect of other companies' shares during the last or current financial year.
Key Developments
Macrilen™ (macimorelin), a ghrelin receptor agonist, is a novel orally-active small molecule that stimulates the secretion of growth hormone. Macrilen™ (macimorelin) has been granted orphan drug designation by the FDA for the evaluation of growth hormone deficiency. The company own the worldwide rights to this novel patented compound. Macrilen™ (macimorelin) is its proposed trade name for macimorelin. The proposed trade name was conditionally approved by the FDA. On December 16, 2016, the company were advised by the EMA that Macrilen™ was rejected as the proposed invented name for macimorelin because of its similarity to the names of other medicines. On March 8, 2018, the company applied for two new invented names for macimorelin: Macrilen ST and Macrilen GHST; however, AEterna Zentaris is also evaluating alternative names given recent feedback received from the EMA.
In late 2016, the company concluded a confirmatory Phase 3 clinical trial of Macrilen™ (macimorelin) for the evaluation of growth hormone deficiency in adults AGHD. The confirmatory trial was an open-label, randomized, two-way crossover study that compared the results of the evaluation of AGHD using Macrilen™ (macimorelin) to the results of the evaluation of AGHD using a procedure known as the "Insulin Tolerance Test" (the "ITT") on the same patients. The trial involved patients, each of whom was evaluated for AGHD using both Macrilen™(macimorelin) and the ITT. Thirty of the patients were evaluated using Macrilen™(macimorelin) a second time to measure the repeatability of the result obtained using Macrilen™ (macimorelin) as the evaluation method. The study population consisted of more than 110 patients who were suspected of having AGHD as a result of the presence of one or more symptoms. This segment of the population included a range of patients from those considered at low risk of having AGHD to those considered at high risk. The study population also included 25 healthy subjects, who had no risk of having AGHD.
On January 4, 2017, the company announced that the confirmatory Phase 3 clinical trial of Macrilen™(macimorelin) failed to achieve its objective of validating a single oral dose of Macrilen™ (macimorelin) for the evaluation of AGHD, using the ITT as a comparator. Based on an analysis of top-line data, Macrilen™ (macimorelin) did not achieve equivalence to the ITT as a means of diagnosing AGHD. Under the study protocol, the evaluation of AGHD with Macrilen™ (macimorelin) would have been considered successful if the lower bound of the two-sided 95% confidence interval for the primary efficacy variables was 75% or higher for "percent negative agreement" with the ITT, and 70% or higher for the "percent positive agreement" with the ITT. While the estimated percent negative agreement met the success criteria, the estimated percent positive agreement did not reach the criteria for a successful outcome. Therefore, the results did not meet the pre-defined equivalence criteria which required success for both the percent negative agreement and the percent positive agreement.
On February 13, 2017, the company announced that, following a comprehensive review of the data obtained from the confirmatory Phase 3 clinical trial of Macrilen™(macimorelin) for the evaluation of AGHD using the ITT as a comparator, the company concluded that Macrilen™(macimorelin) demonstrated performance supportive of FDA registration consideration.
On March 7, 2017, the company announced that the Pediatric Committee of the EMA agreed to its Pediatric Investigation Plan ("PIP") for Macrilen™ (macimorelin) and agreed that the company may defer conducting the PIP until after the company file an MAA seeking marketing authorization for the use of Macrilen™ (macimorelin) for the evaluation of AGHD.
On March 30, 2017, the company announced that, following its meeting with the FDA on March 29, 2017, the company intended to file an NDA seeking approval of MacrilenTM (macimorelin) for the evaluation of AGHD. The announcement also indicated that during its meeting with the FDA, the FDA stated that the clinical studies performed with respect to MacrilenTM (macimorelin) address the prior deficiencies mentioned in the November 5, 2014 complete response letter and that this conclusion paved the way for re-submission by it of an NDA for MacrilenTM (macimorelin). While indicating that the conclusions regarding the performance of MacrilenTM (macimorelin) are review issues subject to an examination of the complete data set, the FDA indicated that the summary data submitted by it prior to the meeting appear to support the propositions advanced by it. Most importantly, the FDA specified the additional statistical analysis of existing data that would be required to further support its conclusions.
On June 30, 2017, the company announced that the company had resubmitted an NDA to the FDA seeking approval of MacrilenTM (macimorelin).
On July 18, 2017, the company announced that the company had been notified by the FDA that its NDA seeking approval of MacrilenTM (macimorelin) for the evaluation of AGHD had been accepted as a complete response to the FDA's November 5, 2014 complete response letter and granted a PDUFA date of December 30, 2017.
On November 27, 2017, the company announced that the MAA for the use of MacrilenTM (macimorelin) for the evaluation of AGHD has been accepted by the EMA for regulatory review. The start of the EMA review procedure for the MAA has been confirmed by EMA as November 23, 2017.
On December 20, 2017, the company announced that the FDA granted marketing approval for MacrilenTM (macimorelin) to be used in the diagnosis of patients with AGHD. On January 17, 2018, the company announced that through AEZS Germany, the company entered into the Strongbridge License Agreement to carry out development, manufacturing, registration and commercialization of Macrilen™ (macimorelin) in the United States and Canada. The company continue to explore various alternatives to monetize its rights to Macimorelin in other countries around the globe.
Business overview
On December 20, 2017, the FDA granted marketing approval for Macrilen™ (macimorelin) to be used in the diagnosis of patients with adult growth hormone deficiency ("AGHD").
Macrilen™ (macimorelin), a ghrelin receptor agonist, is a novel orally-active small molecule that stimulates the secretion of growth hormone. Macrilen™ (macimorelin) has been granted orphan drug designation by the FDA for the evaluation of growth hormone deficiency. The company own the worldwide rights to this novel patented compound. Macrilen™ (macimorelin) is its proposed trade name for macimorelin. The proposed trade name was conditionally approved by the FDA. On December 16, 2016, the company were advised by the EMA that Macrilen™ was rejected as the proposed invented name for macimorelin because of its similarity to the names of other medicines. On March 8, 2018, the company applied for two new invented names for macimorelin: Macrilen ST and Macrilen GHST; however, AEterna Zentaris is also evaluating alternative names given recent feedback received from the EMA.
On January 16, 2018, through AEZS Germany, the company entered into the Strongbridge License Agreement. The company received an upfront cash payment of $24,000,000 from Strongbridge, and, for as long as Macrilen™ (macimorelin) is patent-protected, the Company will be entitled to a 15% royalty on net sales up to $75,000,000 and an 18% royalty on net sales above $75,000,000. Following the end of patent protection in United States or Canada for Macrilen™ (macimorelin), the Company will be entitled to a 5% royalty on net sales in that country. In addition, the Company will also receive one-time payments from Strongbridge following the first achievement of the following commercial milestone events:
$4,000,000 on achieving $25,000,000 annual net sales,$10,000,000 on achieving $50,000,000 annual net sales,$20,000,000 on achieving $100,000,000 annual net sales,$40,000,000 on achieving $200,000,000 annual net sales, and$100,000,000 on achieving $500,000,000 annual net sales.
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the Company will receive a one-time milestone payment of $5,000,000 from Strongbridge.
Strongbridge will fund 70% of the costs of a worldwide pediatric development program to be run by the Company with customary oversight from a joint steering committee. The joint steering committee will be comprised of four persons, two of whom will be appointed by each of Strongbridge and the Company.
In 2017, the company completed a Phase 3 study of the internally developed compound Zoptrex™ (zoptarelin doxorubicin), in the indication for advanced, recurrent endometrial cancer, the results of which study are not supportive to pursue regulatory approval by the FDA. In light of the results of the Zoptrex™ study, its focus has shifted entirely to the commercialization, either directly or through third parties, of Macrilen™ (macimorelin).
The commercial success of Macrilen™ (macimorelin) will depend on several factors, including, but not limited to, the receipt of approvals from the EMA and similar foreign regulatory authorities; developing appropriate distribution and marketing infrastructure and arrangements for its product; launching and growing commercial sales of the product; and acceptance of the product in the medical community, among patients and with third party payers. AEterna Zentaris is not currently conducting any clinical studies.
The company continue to explore various alternatives to monetize its rights to macimorelin in other countries around the globe.
The company also continue to seek opportunities to in-license and acquire products. The company's goal is to become a growth-oriented specialty biopharmaceutical company by pursuing successful development, commercialization and licensing of a product portfolio achieving successful commercial presence and growth, while consistently delivering value to its shareholders, employees and the medical providers and patients who will benefit from its products.
Business Strategy
The company's primary business strategy is to finalize the development, manufacturing, registration and commercialization of Macrilen™ (macimorelin) through the Strongbridge License Agreement in the United States and Canada. The company continue to explore various alternatives to monetize its rights to Macrilen™ (macimorelin) in other countries around the globe, including whether to find other license partners in these jurisdictions or to use its internal resources to commercialize Macrilen™ (macimorelin) in one or more of these countries. The company's vision is to become a growth-oriented specialty biopharmaceutical company.
Macrilen™ (macimorelin)
Macrilen™ (macimorelin) is a novel orally available peptidomimetic ghrelin receptor agonist that stimulates the secretion of growth hormone by binding to the ghrelin receptor (GHSR-1a) and that has potential uses in both endocrinology and oncology indications. Macrilen™ (macimorelin) was granted orphan-drug designation by the FDA for use in evaluating growth hormone deficiency ("GHD").
Competitors for Macrilen™ (macimorelin) as a product for the evaluation of AGHD are principally the diagnostic tests currently performed by endocrinologists, although none of these tests are approved by the FDA for this purpose. The most commonly used diagnostic tests for GHD are:
Measurement of blood levels of Insulin Growth Factor ("IGF")-1, which is typically used as the first test when GHD is suspected. However, this test is not used to definitively diagnose GHD because many growth hormone deficient patients show normal IGF-1 levels.The Insulin Tolerance Test ("ITT"), which has historically been considered the gold standard for the evaluation of AGHD because of its high sensitivity and specificity. However, the ITT is inconvenient to both patients and physicians, administered intravenously (IV), and contra-indicated in certain patients, such as patients with coronary heart disease or seizure disorder, because it requires the patient to experience hypoglycemia to obtain a result. Some physicians will not induce full hypoglycemia, intentionally compromising accuracy to increase safety and comfort for the patient. Furthermore, administration of the ITT includes additional costs associated with the patient being closely monitored by a physician for the two- to four-hour duration of the test and the test must be administered in a setting where emergency equipment is available and where the patient may be quickly hospitalized. The ITT is not used for patients with co-morbidities, such as cardiovascular disease, seizure disorder or a history of brain cancer or for patients who are elderly and frail, due to safety concerns.The Glucagon Stimulation Test ("GST") is considered relatively safe by endocrinologists. The mechanism of action for this test is unclear. Also, this test takes up to three to four hours. It produces side effects in up to one-third of the patients with the most common being nausea during and after the test. This test is administered intramuscularly (IM).The GHRH + ARG test (growth hormone releasing hormone-arginine stimulation) which is an easier test to perform in an office setting and has a good safety profile but is considered to be costly to administer compared to the ITT and the GST. GHRH + ARG is approved in the EU and has been proposed to be the best alternative to ITT, but GHRH is no longer available in the United States. This test is administered intravenously (IV).
Oral administration of Macrilen™ (macimorelin) offers convenience and simplicity over the current GHD tests used, all of which require either intravenous or intramuscular administration. Additionally, Macrilen™ (macimorelin) may demonstrate a more favorable safety profile than existing diagnostic tests, some of which may be inappropriate for certain patient populations, e.g. diabetes mellitus or coronary heart disease, and have demonstrated a variety of side effects, which Macrilen™ (macimorelin) has not thus far. These factors may be limiting the use of GHD testing and may potentially enable Macrilen™ (macimorelin) to become the product of choice in evaluating AGHD. The company believe that Macrilen™ (macimorelin) is likely to rapidly displace the ITT as the preferred means of evaluating AGHD for the following reasons:
it is safer and more convenient than the ITT because it does not require the patient to become hypoglycemic;Macrilen™ (macimorelin) is administered orally, while the ITT requires an intravenous injection of insulin;Macrilen™ (macimorelin) is a more robust test than the ITT leading to evaluable test results;Macrilen™ (macimorelin) results are highly reproducible;the evaluation of AGHD using Macrilen™ (macimorelin) is less time-consuming and labor-intensive than the ITT; andthe evaluation can be conducted in the physician's office rather than in a hospital-like setting.
The company believe that approximately 60,000 AGHD tests will be conducted annually, in the U.S, after the introduction of Macrilen™ (macimorelin). In addition, based on published information from the U.S. Centers for Disease Control and Prevention, different scientific publications and Navigant Research, the company estimate that the total potential U.S. market for AGHD evaluation is approximately 150,000 tests per year, including the evaluation of patients who have suffered traumatic brain injury ("TBI"). In patients with TBI, GHD is frequent and may contribute to cognitive sequelae and reduction in quality of life. GHD may develop in approximately 19% of both severe and moderate hospitalized TBI victims.
Development History
The following is a summary of the history of its development of Macrilen™ (macimorelin):
The company out-licensed the development compound macimorelin acetate to Ardana Bioscience in 2004. Ardana Bioscience subsequently initiated the clinical development program of macimorelin acetate as an orally active compound intended to be used in the diagnosis of AGHD. Following agreement with the FDA on the study design, Ardana Bioscience initiated a pivotal Phase 3 study in 2007, which tested the compound compared to a test of growth hormone- releasing hormone ("GHRH") + L-Arginine ("ARG"), using a competitor's compound. The study was discontinued in 2008 due to Ardana Bioscience's bankruptcy. The company terminated Ardana Bioscience's license to the compound due to its bankruptcy.On October 19, 2009, the company announced that the company had initiated activities intended to complete the clinical development of Macrilen™ (macimorelin) for use in evaluating AGHD. The company had already assumed the sponsorship of the Investigational New Drug Application ("IND") from Ardana Bioscience and discussed with the FDA the best way to complete the ongoing Phase 3 clinical trial and subsequently to file an NDA for approval of Macrilen™ (macimorelin) for use in evaluating AGHD. The pivotal Phase 3 trial was designed to investigate the safety and efficacy of the oral administration of Macrilen™ (macimorelin) as a growth hormone stimulator for use in evaluating AGHD. It was accepted by the FDA that for the ongoing part of the study, Macrilen™ (macimorelin) would not be compared to the GHRH + ARG test because the competitor's compound had been removed from the market.On December 20, 2010, the company announced the company had reached agreement with the FDA on a Special Protocol Assessment ("SPA") for Macrilen™ (macimorelin), enabling it to complete the ongoing registration study required to gain approval for use in evaluating AGHD. The first part of the study, conducted by its former licensee, Ardana, was a two-way cross-over study and included 43 patients with confirmed AGHD or multiple pituitary hormone deficiencies and a low IGF-1. A control group of ten subjects without AGHD was matched to patients for age, gender, body mass index and (for females) estrogen status.On July 26, 2011, the company announced the completion of the Phase 3 study of Macrilen™ (macimorelin) as a first oral product for use in evaluating AGHD and the decision to meet with the FDA for the future filing of an NDA for the registration of Macrilen™ (macimorelin) in the United States.On June 26, 2012, the company announced that the final results from a Phase 3 trial for Macrilen™ (macimorelin) showed that the drug is safe and effective in evaluating AGHD. Jose M. Garcia, MD, PhD, then of the Baylor College of Medicine and the Michael E. DeBakey VA Medical Center, disclosed these data during an oral presentation at the 94th ENDO Annual Meeting and Expo in Houston, Texas. The study had originally been designed as a cross-over trial of Macrilen™ (macimorelin) compared to the GHRH + ARG test in AGHD patients and in controls matched for body mass index ("BMI"), estrogen status, gender and age. After 43 AGHD patients and ten controls had been tested, the GHRH + ARG test became unavailable because the competitor's compound was withdrawn from the market. The study was completed by testing ten more AGHD patients and 38 controls with Macrilen™ (macimorelin) alone. Of the 53 AGHD subjects enrolled, 52 received Macrilen™ (macimorelin), and 50 who had confirmed AGHD prior to study entry were included in this analysis, along with 48 controls. Two AGHD subjects could not be matched due to the combination of young age, high BMI and estrogen use. The objective of this clinical trial was to determine the efficacy and safety of Macrilen™ (macimorelin) in the evaluation of AGHD. Mean peak growth hormone ("GH") levels in AGHD patients and controls following Macrilen™ (macimorelin) administration were 2.36ng/mL (range 0.03-33) and 17.71ng/mL (range 10.5-94), respectively. The ROC plot analysis yielded an optimal GH cut-point of 2.7ng/mL, with 82% sensitivity, 92% specificity and a 13% misclassification rate. Obesity (BMI>30) was present in 58% of cases and controls, and peak GH levels were inversely associated with BMI in controls. Adverse events ("AE") were seen in 37% of AGHD patients and in 21% of controls following Macrilen™ (macimorelin). In contrast, 61% of AGHD subjects and 30% of controls experienced AEs with L ARG+GHRH. The most common AEs after Macrilen™ (macimorelin) were unpleasant taste (19.2%) and diarrhea (3.8%) for the AGHD patients and unpleasant taste (4.2%) and diarrhea (4.2%) for the matched controls. No clinically meaningful changes from baseline in ECG results during the study for AGHD patients were observed; however, one control subject had an ECG change (T wave abnormality and QTc interval prolongation) one hour after treatment with Macrilen™ (macimorelin) that was considered a serious treatment-related adverse event and resolved spontaneously within 24 hours. The subject had been pre-treated with citalopram, a drug that was later reported by the FDA to be associated with QT prolongation, although the patient had stopped this medication seven days prior to dosing. In an expert statement of January 9, 2015, Prof. Dr. W. Haverkamp, Centrum Herz-, Kreislauf- und Gefäßmedizin, Charité, Berlin, considered the observed QT prolongation to be not related to Macrilen™ (macimorelin). Overall, this study demonstrated that Macrilen™ (macimorelin) is safe and effective for use in evaluating AGHD.In November 2013, the company filed an NDA for Macrilen™ (macimorelin) for the evaluation of AGHD by evaluating the pituitary gland secretion of growth hormone in response to an oral dose of the product. The FDA accepted the NDA for substantive review in January 2014. On November 6, 2014, the FDA informed it, by issuing a Complete Response Letter ("CRL"), that it had determined that its NDA could not be approved in its then present form. The CRL stated that the planned analysis of its pivotal trial did not meet its stated primary efficacy objective as agreed to in the SPA. The CRL further mentioned issues related to the lack of complete and verifiable source data for determining whether patients were accurately diagnosed with AGHD. The FDA concluded that, "in light of the failed primary analysis and data deficiencies noted, the clinical trial does not by itself support the indication." To address the deficiencies identified above, the CRL stated that the company needed to demonstrate the efficacy of Macrilen™ (macimorelin) as a diagnostic test for GHD in a new, confirmatory clinical study. The CRL also stated that a serious event of electrocardiogram QT interval prolongation occurred for which attribution to drug could not be excluded. Therefore, a dedicated thorough QT study to evaluate the effect of macimorelin on the QT interval would be necessary.Following receipt of the CRL, the company assembled a panel of experts in the field of growth-hormone deficiency, including experts in the field from both the United States and the EU. The panel met on January 8, 2015, during which the company discussed its conclusions from the CRL, as well as the potential design of a new pivotal study. The panel advised it to continue to seek approval for Macrilen™ (macimorelin) because of their confidence in its efficacy and because there currently is no FDA-approved diagnostic test for AGHD. In parallel, the company collected information on timelines and costs for such a study.During an end-of-review meeting with the FDA on March 6, 2015, the company agreed with the FDA on the general design of the confirmatory Phase 3 study of Macrilen™ (macimorelin) for the evaluation of AGHD, as well as evaluation criteria. The company agreed with the FDA that the confirmatory study will be conducted as a two-way crossover with the ITT as the benchmark comparator.On April 13, 2015, the company announced plans to conduct a new, confirmatory Phase 3 clinical study to demonstrate the efficacy of Macrilen™ (macimorelin) for the evaluation of AGHD, as well as a dedicated thorough QT study to evaluate the effect of Macrilen™ (macimorelin) on myocardial repolarization. The confirmatory Phase 3 clinical study of Macrilen™ (macimorelin), entitled "Confirmatory validation of oral macimorelin as a growth hormone (GH) stimulation test (ST) for the diagnosis of AGHD in comparison with the insulin tolerance test (ITT)", was designed as a two-way crossover study with the ITT as the benchmark comparator and involved 31 sites in the United States and Europe. The study population was planned to include at least 110 subjects (at least 55 ITT-positive and 55 ITT-negative) with a medical history documenting risk factors for AGHD, and was planned to include a spectrum of subjects from those with a low risk of having AGHD to those with a high risk of having the condition.On May 26, 2015, the company announced that the company had received written scientific advice from the EMA regarding the further development plan, including the study design, for the new confirmatory Phase 3 clinical study of Macrilen™ (macimorelin) for use in evaluating AGHD. As a result of the advice, the company believe that the confirmatory Phase 3 study that was agreed with the FDA meets the EMA's study-design expectations as well, allowing for U.S. and European approval, if the study is successful.On November 19, 2015, the company announced the enrollment of the first patient in the confirmatory Phase 3 clinical study of Macrilen™ (macimorelin).On October 26, 2016, the company announced completion of patient recruitment for the confirmatory Phase 3 clinical trial of Macrilen™ (macimorelin) as a growth hormone stimulation test for the evaluation of AGHD.The dedicated thorough QT study to evaluate the effect of macimorelin on the QT interval, as requested by the FDA in the CRL, was conducted and completed in 2016.On January 4, 2017, the company announced that, based on an analysis of top-line data, the confirmatory Phase 3 clinical trial of Macrilen™ (macimorelin) failed to achieve one of its co-primary endpoints. Under the study protocol, the evaluation of AGHD with Macrilen™ (macimorelin) would be considered successful, if the lower bound of the two-sided 95% confidence interval for the primary efficacy variables was 75% or higher for "percent negative agreement" with the ITT, and 70% or higher for the "percent positive agreement" with the ITT. While the estimated percent negative agreement met the success criteria, the estimated percent positive agreement did not reach the criteria for a successful outcome. Therefore, the results did not meet the pre-defined equivalence criteria which required success for both the percent negative agreement and the percent positive agreement.On February 13, 2017, the company announced that, after reviewing the raw data on which the top-line data were based, the company had concluded that Macrilen™ (macimorelin) had demonstrated performance supportive of achieving FDA registration and that the company intended to pursue registration. The announcement set forth the facts on which its conclusion was based. The Company met with the FDA at the end of March 2017 to discuss this position.On March 7, 2017, the company announced that the Pediatric Committee ("PDCO") EMA agreed to the Company's Pediatric Investigation Plan ("PIP") for Macrilen™ (macimorelin) and agreed that the Company may defer conducting the PIP until after it files a Marketing Authorization Application ("MAA") seeking marketing authorization for the use of Macrilen™ (macimorelin) for the evaluation of AGHD.On July 18, 2017, the company were provided a PDUFA date of December 30, 2017 by the FDA.On November 27, 2017, the EMA accepted its MMA submission for Macrilen™ (macimorelin).On December 20, 2017, the FDA approved the market authorization for Macrilen™ (macimorelin), to be used in the diagnosis of patients with adult growth hormone deficiency (AGHD).On March 23, 2018, the company received from the EMA a Day 120 List of Questions, which was issued in connection with its MMA submission for Macrilen™ (macimorelin). AEterna Zentaris is in the process of reviewing.
Zoptrex™
ZoptrexTM is a complex molecule that combines a synthetic peptide carrier with doxorubicin, a well-known chemotherapy agent. The synthetic peptide carrier is a luteinizing hormone-releasing hormone ("LHRH") agonist, a modified natural hormone with affinity for the LHRH receptor. The design of the compound allows for the specific binding and selective uptake of the cytotoxic conjugate by LHRH receptor-positive tumors.
On January 30, 2017, the company announced the completion of the clinical phase of the pivotal Phase 3 ZoptEC (Zoptarelin Doxorubicin in Endometrial Cancer) study with the occurrence of the 384th death.
On May 1, 2017, the company announced that the ZoptEC pivotal Phase 3 clinical study of Zoptrex™ (zoptarelin doxorubicin) in women with locally advanced, recurrent or metastatic endometrial cancer did not achieve its primary endpoint of demonstrating a statistically significant increase in the median period of overall survival of patients treated with Zoptrex™ (zoptarelin doxorubicin) as compared to patients treated with doxorubicin. The results of the study are not supportive to pursue regulatory approval by the FDA. Based on this outcome, the company do not anticipate conducting clinical trials of Zoptrex™ (zoptarelin doxorubicin) with respect to any other indications. The company also discontinued the development of AEZS-138/Disorazol Z, as it was based on the same concept as Zoptrex™ (zoptarelin doxorubicin).
AEterna Zentaris has licensed the development, commercialization and certain other rights to Zoptrex™ to Sinopharm A-Think for China, Hong Kong and Macau; to an affiliate of Orient EuroPharma Co., Ltd. for Taiwan and southeast Asia; to Rafa Laboratories, Ltd for Israel and the Palestinian territories and to Specialised Therapeutics Asia Pte Ltd for Australia and New Zealand.
Overview of its Commercial Operations
The company's commercial operations were significantly reduced in the fourth quarter of 2017. The company eliminated its contract sales team in its entirety, as well as remaining sales management in November 2017, in accordance with the terms of its agreement with inVentiv Commercial Services, LLC, an affiliate of inVentiv Health, Inc. ("inVentiv"), a contract-sales organization. The company's agreement with inVentiv commenced in November 2014.
Pursuant to termination of the inVentiv agreement, the company ended its co-promotion with EMD Serono, Inc. ("EMD Serono") and Armune BioScience, Inc. ("Armune").
Until September 1, 2016, the company co-promoted a product, EstroGel®, and until termination of its sales team in November 2017, the inVentiv sales force promoted two products:
Saizen® [somatropin (rDNA origin) for injection] is a prescription medicine indicated for the treatment of growth hormone deficiency in children and adults. The company promoted Saizen® pursuant to its promotional services agreement (the “EMD Serono Agreement”) with EMD Serono Inc. (“EMD Serono”), which the company entered into in May 2015 and amended as of December 31, 2016. The EMD Serono Agreement, as amended, provided that the company were to promote Saizen® in specific agreed-upon U.S. territories to adult and pediatric endocrinologists in exchange for a sales commission that was based upon new patient starts of the product. The agreement was terminated in accordance with its terms in December 2017.
APIFINY® is the only cancer-specific, non-PSA blood test for the evaluation of the risk of prostate cancer. The test was developed by Armune BioScience, Inc. (“Armune”), a medical diagnostics company that develops and commercializes unique proprietary technology exclusively licensed from the University of Michigan for diagnostic and prognostic tests for cancer. The company entered into a co-marketing agreement with Armune in November 2015 (the “Armune Agreement”), which was amended effective as of June 1, 2016, which allowed it to exclusively promote APIFINY® throughout the entire United States. The company received a commission for each test performed resulting from its targeted promotion without regard to any established baseline. The Armune Agreement, as amended, had a three-year term that renewed automatically for successive one-year periods. The parties agreed in January 2018 that the Armune Agreement was terminated.
On December 20, 2017, the company received FDA approval for Macrilen™ (macimorelin) indicated for the diagnosis of AGHD. Following a detailed review process undertaken by a committee of its independent directors, the company entered into the Strongbridge License Agreement to carry out development, manufacturing, registration and commercialization of Macrilen™ (macimorelin) in the United States and Canada. The company continue to explore various alternatives to monetizing rights to macimorelin in other countries around the globe, including whether to find other license partners in these jurisdictions or to use its internal resources to commercialize in certain of these countries.
Under the Strongbridge License Agreement, the company received an upfront cash payment of $24,000,000, and, for as long as Macrilen™ (macimorelin) is patent-protected, the company will be entitled to a 15% royalty on net sales up to $75,000,000 and an 18% royalty on net sales above $75,000,000. Following the end of patent protection in United States or Canada for Macrilen™ (macimorelin), the company will be entitled to a 5% royalty on net sales in these countries. In addition, the company also will receive one-time payments from Strongbridge following the first achievement of the following commercial milestone events:
$4,000,000 on achieving $25,000,000 annual net sales$10,000,000 on achieving $50,000,000 annual net sales$20,000,000 on achieving $100,000,000 annual net sales$40,000,000 on achieving $200,000,000 annual net sales$100,000,000 on achieving $500,000,000 annual net sales
Upon approval by the FDA of a pediatric indication for Macrilen™ (macimorelin), the company will receive a one-time milestone payment of $5,000,000 from Strongbridge.
Strongbridge will fund 70% of the costs of a worldwide pediatric development program to be run by the Company with customary oversight from a joint steering committee. The joint steering committee will be comprised of four persons, two of whom will be appointed by each of Strongbridge and the Company.
A description of the principal geographic areas in which the company compete, including a geographical and categorical breakdown of its revenues in the past three years is presented in note 23 (Segment information) to its consolidated financial statements included in this Annual Report on Form 20-F at Item 18.
Seasonality
As a specialty biopharmaceutical company, the Company does not consider any of its products or services to be seasonal.
Raw Materials
Raw materials and supplies are generally available in quantities adequate to meet the needs of its business. The company will be dependent on third-party manufacturers for the pharmaceutical products that the company will market. An interruption in the availability of certain raw materials or ingredients, or significant increases in the prices paid by it for them, could have a material adverse effect on its business, financial condition, liquidity and operating results.
Intellectual Property - Patents
The company seek to protect its compounds, manufacturing processes, compositions and methods of medical use for its lead drugs and drug candidates through a combination of patents, trade secrets and know-how. The company's patent portfolio consists of approximately 12 owned and in-licensed patent families (issued, granted or pending in the United States, Europe and other jurisdictions). The patent positions of companies in the biotechnology and pharmaceutical industries are highly uncertain and involve complex legal and factual questions. Therefore, the company cannot predict the breadth of claims, if any, that may be allowed under any of its patent applications, or the enforceability of any of its allowed patents.
Patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depends on the type of patent, the scope of its coverage and the availability of legal remedies in the country. In the United States, the patent term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent, in which the patentee may file an application for yearly interim extensions within five years if the patent will expire and the FDA has not yet approved the NDA. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended.
Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In these jurisdictions, however, no interim extensions exist and the marketing approval must be granted before the patent expires. In the future, if and when its pharmaceutical products receive FDA approval, the company expect to apply for patent term extensions on patents covering those products. While the company anticipate that any such applications for patent term extensions will likely be granted, the company cannot predict the precise length of time for which such patent terms would be extended in the United States, Europe or other jurisdictions. If AEterna Zentaris is not able to secure patent term extensions on patents covering its products for meaningful periods of additional time, the company may not achieve or sustain profitability, which would adversely affect its business.
In addition to patent protection, its products may benefit from the market-exclusivity provisions contained in the orphan-drug regulations or the pediatric-exclusivity provisions or other provisions of the FDA Act, such as new chemical entity exclusivity or new formulation exclusivity. Orphan drug regulations provide incentives to pharmaceutical and biotechnology companies to develop and manufacture drugs for the treatment of rare diseases, currently defined as diseases that exist in fewer than 200,000 individuals in the U.S., or diseases that affect more than 200,000 individuals in the U.S. but that the sponsor does not realistically anticipate will generate a net profit. Under these provisions, a manufacturer of a designated orphan drug can seek tax benefits, and the holder of the first FDA approval of a designated orphan product will be granted a seven-year period of marketing exclusivity for such FDA-approved orphan product. In the U.S., the FDA has the authority to grant additional data protection for approved drugs where the sponsor conducts specified testing in pediatric or adolescent populations. If granted, this pediatric exclusivity provides an additional six months which are added to the term of data protection as well as to the term of any relevant patents, to the extent these protections have not already expired. The company may also seek to utilize market exclusivities in other territories, such as in the EU. There can be no assurance that any of its drug candidates will obtain such orphan drug designation, pediatric exclusivity, new chemical entity exclusivity or any other market exclusivity in the U.S., the EU or any other territory, or that the company will be the first to receive the regulatory approval in a given country or territory for such drugs so as to be eligible for any market exclusivity protection.
Zoptrex™
AEterna Zentaris has licensed the intellectual property and associated rights relating to LHRH agonists and LH-RH antagonists carrying various cytotoxic radicals (including zoptarelin doxorubicin) from the Administrators of the Tulane Educational Fund ("Tulane") pursuant to a license agreement dated September 17, 2002 between Tulane, as licensor, and AEZS GmbH, as licensee (the "Tulane Agreement"). The Tulane Agreement grants to it an exclusive worldwide license for all therapeutic uses of LH-RH agonists and LH-RH antagonists carrying various cytotoxic radicals, to the extent covered by one of the patents listed below. The term of the Tulane Agreement continues for ten years after the first commercial sale of a product based on the licensed intellectual property (a "Licensed Product") or until the expiration of the last to expire of the patents listed below, whichever is longer, on a country-by- country basis.
Pursuant to the Tulane Agreement, AEterna Zentaris is required to pay Tulane the following amounts: ![]() $400,000 upon the first grant of regulatory approval for a Licensed Product in the U.S., Canada, the EU or Japan; (ii) 10% of all consideration received by it from a sublicensee for authorization to use the licensed intellectual property to develop, manufacture, market, distribute and sell a Licensed Product; (iii) 2.5% of its net sales of Licensed Products; and (iv) 50% of any royalties that the company receive from a sublicensee with respect to its net sales of Licensed Products; provided, however, that the payment with respect to royalties received from a sublicensee shall not be less than 1.75% nor more than 2.5% of the sublicensee's net sales of the Licensed Product. The following patents are covered by the Tulane Agreement:
$400,000 upon the first grant of regulatory approval for a Licensed Product in the U.S., Canada, the EU or Japan; (ii) 10% of all consideration received by it from a sublicensee for authorization to use the licensed intellectual property to develop, manufacture, market, distribute and sell a Licensed Product; (iii) 2.5% of its net sales of Licensed Products; and (iv) 50% of any royalties that the company receive from a sublicensee with respect to its net sales of Licensed Products; provided, however, that the payment with respect to royalties received from a sublicensee shall not be less than 1.75% nor more than 2.5% of the sublicensee's net sales of the Licensed Product. The following patents are covered by the Tulane Agreement:
U.S. patent 5,843,903 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expired in November 2015.European patent 0 863 917 B1 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expired in November 2016.Japanese patent 3 987 575 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expired in November 2016.Chinese patent ZL96198605.0 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expired in November 2016.Hong Kong patent 1017363 covers zoptarelin doxorubicin and other related targeted cytotoxic anthracycline analogs, pharmaceutical compositions comprising the compounds as well as their medical use for the treatment of tumors. This patent expired in November 2016.In early 2015, the company filed a European patent application directed to a novel method of manufacturing Zoptrex™. Within the 12 months priority period, the company also filed an international patent application for the manufacturing process, as well as national patent applications in selected countries, including the U.S., China, and Taiwan, Japan and India. The company decided to file patent applications in additional territories after the European Patent Office issued a search report for the European patent application that the company consider to be favorable. The claimed manufacturing process is expected to result in a significant reduction in its cost of manufacturing Zoptrex™, providing it with what should be a stronger competitive position and discouraging competition from generic manufacturers after its five-year period of data exclusivity expires.
Macrilen™ (macimorelin):
The company hold the worldwide rights to macimorelin pursuant to an exclusive license agreement with the French Centre National de la Recherche Scientifique, as licensor, and AEZS GmbH, as licensee.
The following patents relate to Macrilen™ (macimorelin):
U.S. patent 6,861,409 covers Macrilen™ (macimorelin) and U.S. patent 7,297,681 covers other related growth hormone secretagogue compounds, each also covering pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. U.S. patent 6,861,409 and U.S. patent 7,297,681 both expire in August 2022.European patent 1 289 951 covers Macrilen™ (macimorelin) and European patent 1 344 773 covers other related growth hormone secretagogue compounds, pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. EP patent 1 289 951 and EP patent 1 344 773 both expire in June 2021.Japanese patent 3 522 265 covers Macrilen™ (macimorelin) and pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. This patent expires in June 2021.anadian patent 2,407,659 covers Macrilen™ (macimorelin) and pharmaceutical compositions comprising the compounds as well as their medical use for elevating the plasma level of growth hormone. This patent expires in June 2021.U.S. patent 8,192,719 covers a method of assessing pituitary-related growth hormone deficiency in a human or animal subject comprising an oral administration of the compound Macrilen™ (macimorelin) and determination of the level of growth hormone in the sample and assessing whether the level of growth hormone in the sample is indicative of growth hormone deficiency. This patent expires in October 2027.European patent 1 984 744 covers a method of assessing pituitary-related growth hormone deficiency by oral administration of Macrilen™ (macimorelin). This patent expires in February 2027.Japanese patent 4 852 728 covers a method of assessing pituitary-related growth hormone deficiency by oral administration of Macrilen™ (macimorelin). This patent expires in February 2027.
Disorazol Z - LHRH conjugates (AEZS-138):
The company own a number of patents that relate to its Disorazol Z - LHRH conjugates, as follows:
U.S. patent 7,741,277 covers AEZS-138 (disorazol Z - LHRH conjugate). This patent expires in January 2028 (including PTA).U.S. patent 8,470,776 covers methods of treatment for compound AEZS-138 (disorazol Z - LHRH conjugate). This patent expires in February 2029 (including PTA).European patent application 2,066,679 covers AEZS-138 (disorazol Z - LHRH conjugate) as well as methods of treatment for this compound. If granted, this patent will expire in September 2027.Japanese patent 5,340,155 covers AEZS-138 (disorazol Z - LHRH conjugate) as well as methods of treatment for this compound. This patent expires in September 2027.
Organizational structure
The company's corporate structure, the jurisdiction of incorporation of its direct and indirect subsidiaries and the percentage of shares that the company held in those subsidiaries as at December 31, 2017 is depicted in the chart set forth under the caption "Item 4.A. History and development of the Company".
| Location | Use of space | Square Footage | Type of interest |
|---|---|---|---|
| 315 Sigma Drive, Summerville SC 29486, Weismüllerstr. 50 | Partially occupied for management, administration, commercial operations and business development | 300 | Leasehold |
| Weismüllerstr. 50, D-60314, Frankfurt-am-Main, Germany | Occupied for management, R&D, business development and administration | 36168 | Leasehold |
The company believe that its current facilities are adequate to meet its ongoing needs and that, if the company require additional space, the company will be able to obtain additional facilities on commercially reasonable terms.




