Adamas Pharmaceuticals
Adamas Pharmaceuticals, Inc (ADMS)., seek to redefine the treatment experience for patients suffering from chronic neurological diseases. The company's vision is grand, its goal bold: to create and commercialize a new generation of medicines intended to lessen the burden of disease on patients, caregivers and society. With a new commercial medicine and robust pipeline of investigational programs focused on meaningfully differentiated treatment options for patients, the company believe Adamas Pharmaceuticals is well on its way. The company's therapeutic targets include a broad range of neurologic diseases, including Parkinson’s disease, multiple sclerosis, epilepsy and Alzheimer’s disease.
The company's treatment innovations stem from a deep scientific understanding of time-dependent biology – the deliberate mapping of disease patterns and drug activity – along with a goal to meaningfully increase the efficacy of known molecules without compromising tolerability. This approach is designed to ensure that its medicines fit within, rather than define, people’s daily lives. The company's goal is to develop medicines that are timed for the benefit of patients.
The company's understanding of time-dependent biological processes informs its every innovation, targeting advancement in treatment of chronic neurologic disorders. The company's expanding portfolio includes:
Approved Product:
GOCOVRI TM (amantadine) extended release capsules, formerly referred to as ADS-5102, for the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy, with or without concomitant dopaminergic medications. GOCOVRI was approved for marketing by the U.S. Food and Drug Administration, or FDA, on August 24, 2017, with seven years of orphan exclusivity and additional patent protections, and the company fully launched GOCOVRI with a deployed sales force in January 2018.
Potential Additional Indications for GOCOVRI (amantadine) Extended Release Capsules (ADS-5102):
ADS-5102 in development for the treatment of walking impairment in patients with multiple sclerosis. The company expect the start of its Phase 3 pivotal study in this supplemental indication to occur early in the second quarter of 2018.ADS-5102 in research and potential development for additional indications, including the treatment of wearing OFF and delaying motor complications in Parkinson’s disease, tardive dyskinesia, Huntington’s chorea, Tourette syndrome, and non-motor disorders, including depression, and anti-psychotic induced weight gain. The company expect to select additional indications for ADS-5102 by first quarter 2019.
Product Candidates:
A- DS-4101 (lacosamide) modified release capsules in development for the treatment of partial onset seizures in patients with epilepsy. Adamas Pharmaceuticals has requested a meeting with the FDA in the first half of 2018, with the start of a Phase 3 pivotal study planned for 2019, depending on FDA feedback.
Additional product candidates in research based on potential new discoveries in Parkinson’s disease, multiple sclerosis, epilepsy, as well as new research programs in psychiatry.
Partnered Products:
Namzaric ® (memantine hydrochloride extended release and donepezil hydrochloride) capsules for the treatment of moderate to severe dementia of an Alzheimer’s type, marketed in the United States by Allergan plc under an exclusive license agreement between it and Forest Laboratories Holdings Limited (“Forest”), an indirect wholly-owned subsidiary of Allergan plc. Namenda XR ® (memantine hydrochloride) extended release capsules for the treatment of moderate to severe dementia of an Alzheimer’s type, marketed in the United States by Allergan plc under the Forest license agreement.
Products in its wholly-owned portfolio, potential additional indications for these products, and its product candidates, are protected by an array of intellectual property, including robust and diversified patent claims, and regulatory exclusivities. For example, GOCOVRI is protected by seven-year orphan drug exclusivity, three-year new product exclusivity, and issued patents and pending patent applications out to at least 2035.
Adamas Pharmaceuticals has developed its current portfolio of therapies in a capital efficient manner. As of December 31, 2017 , the company had raised a total of $201.3 million from equity financings, including $61.8 million in net proceeds raised in January 2016 from the sale of 2,875,000 shares of common stock. The company also received $160.0 million in upfront and milestone payments and $4.1 million in development funding from its partnership with Allergan plc. As of December 31, 2017 , the company had an accumulated deficit of $211.7 million and $176.4 million in cash, cash equivalents, and investments. In May 2017, the company entered into a Royalty-Backed Loan, or HCRP Loan, with HealthCare Royalty Partners (“HCRP”). As of December 31, 2017 , long-term debt related to its HCRP Loan was $102.6 million . In January 2018, the company raised an additional $134.1 million in net proceeds from the sale of 3,450,000 shares of common stock.
Market Opportunity
The company estimate that approximately 36 million people in the United States suffer from chronic central nervous system, or CNS, disorders such as Parkinson’s disease, multiple sclerosis, epilepsy, psychosis, depression, and Alzheimer’s disease. CNS diseases are frequently treated with multiple medications having different mechanisms of action with the goal of maximizing symptomatic benefits for patients. Existing CNS drugs often require frequent dosing and may have tolerability issues that limit the amount of the drug that can be taken each day. The company believe that many CNS disorders could be better addressed in individual patients, as well as society as a whole, if drug concentrations (or the pharmacokinetic profiles) were shaped as a function of time and disease activity, to improve treatment efficacy while maintaining tolerability.
Strategy
The company's business strategy is to discover, develop, and commercialize clinically differentiated medicines for patients suffering from chronic neurologic disorders, based upon its understanding of time-dependent biology.
Portfolio
The following table summarizes its portfolio:
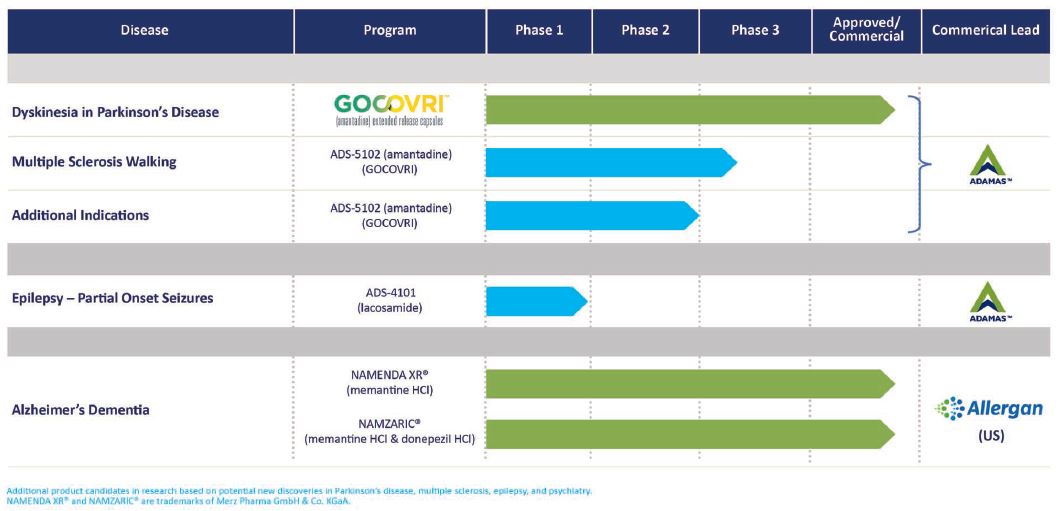
Approved Product:
GOCOVRI (formerly referred to as ADS-5102) for the Treatment of Dyskinesia in Patients with Parkinson’s Disease
GOCOVRI TM (amantadine) extended-release capsules is the first and only medicine approved by the FDA for the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy, with or without concomitant dopaminergic medications. GOCOVRI, a high-dose, 274-mg amantadine taken once-daily at bedtime that delivers high levels of amantadine in the morning and throughout the day when dyskinesia occurs, was approved by the FDA on August 24, 2017, and was granted seven years of orphan exclusivity upon its approval. GOCOVRI is now available for patients in need, and Adamas Pharmaceuticals is actively educating physicians about the GOCOVRI proven efficacy and safety profile, and promoting to physicians. The company made GOCOVRI available for physician and patient use in the fourth quarter of 2017, and commenced the full commercial launch of GOCOVRI in January 2018. In addition to orphan exclusivity that protects GOCOVRI into 2024, issued patents and filed patent applications potentially provide GOCOVRI additional protections through at least 2035.
Parkinson’s disease is a chronic, neurodegenerative disorder affecting close to one million people in the United States. Levodopa, which replaces lost dopamine, is considered the “gold standard” and the most effective therapy for Parkinson’s disease. Over time, people with Parkinson’s disease require increasingly higher or more frequent doses of levodopa to avoid recurrent periods of OFF time – characterized by slowness of movement, rigidity, impaired walking, tremors, and postural instability – when the underlying symptoms of Parkinson’s disease return. At this stage of the Parkinson’s disease journey, it is characterized by an over-activated glutamate system, which leads to the symptoms of dyskinesia and OFF time. Accordingly, as Parkinson’s disease progresses, approximately 90 percent of people on levodopa therapy will experience dyskinesia, which is characterized by involuntary movements that are non-rhythmic, purposeless and unpredictable, impacting peoples’ daily lives. Of the 400,000 Parkinson’s disease patients in the United States with motor complications, approximately 150,000 to 200,000 suffer with dyskinesia.
In a robust clinical program consisting of three randomized, placebo-controlled studies and a two-year, ongoing, open label safety study, GOCOVRI demonstrated a durable reduction in dyskinesia and secondarily in OFF time in people with Parkinson’s disease. Specifically, the pooled data analysis from the two positive, Phase 3 pivotal trials of GOCOVRI demonstrates:
A 41% reduction in dyskinesia as measured on the Unified Dyskinesia Rating Scale total score, compared to 14% for placebo at week 12;A reduction in OFF time of approximately one hour per day (placebo adjusted); andAn increase of approximately 4.0 hours in functional time daily (or ON time without troublesome dyskinesia).
The most common adverse reactions with GOCOVRI were hallucinations, dizziness, dry mouth, peripheral edema, constipation, falls and orthostatic hypotension. Warnings and precautions with GOCOVRI include falling asleep during activities of daily living, suicidality and depression, orthostatic hypertension/dizziness, and hallucinations/psychotic behavior.
In addition, the ongoing open label safety study of GOCOVRI has demonstrated the long-term durability and safety of GOCOVRI out to 88 weeks, and a significant improvement in patients in the study who were switched from amantadine immediate release treatment to GOCOVRI. The company expect the final results of the two-year open label safety study to be reported mid-year in 2018.
As of December 31, 2017, after being available for a little over two months for physician and patient use without sales-based promotional efforts by it, 100 distinct prescribers had prescribed GOCOVRI for Parkinson’s disease patients with dyskinesia. This early reception of GOCOVRI by physicians is consistent with its market research indicating that GOCOVRI would be well-received by physicians, patients and payers. In that research, almost 60% of physicians agree that “efficacy in reducing dyskinesia” was the most important unmet need for dyskinesia treatment. On January 8, 2018, the company deployed its field sales team of 59 experienced neurology account specialists specifically to drive awareness and promote GOCOVRI to approximately 6,500 physicians who treat Parkinson’s disease patients. The company's sales team will be educating physicians about appropriate use of GOCOVRI using marketing materials developed with feedback from experts in the Parkinson’s disease community, as well as the published, peer reviewed scientific articles reporting the data from its two pivotal Phase 3 studies of GOCOVRI.
Additionally, while GOCOVRI faces similar launch challenges as any newly approved medicine with a new indication, Adamas Pharmaceuticals is pleased with the payer response to GOCOVRI’s availability since October 2017 at a list price of $28,500 per year. To further facilitate patient access, in October 2017, the company launched GOCOVRI Onboard, a patient services program. GOCOVRI Onboard provides reimbursement assistance as well as a Quick Start program that offers eligible patients a supply of GOCOVRI within days of receiving a prescription, while insurance coverage is being adjudicated; a co-pay assistance program for commercially insured patients, to ensure that they pay no more than $20 per prescription; a patient assistance program for under insured or non-insured patients; and the provision of information for government insured patients about available programs to assist with their out of pocket costs.
Potential Additional Indications for GOCOVRI (amantadine) extended release capsules (formerly ADS-5102):
ADS-5102 in Development for the Treatment of Walking Impairment in Patients with Multiple Sclerosis
ADS-5102, a high-dose amantadine investigational agent taken once-daily at bedtime, was designed to provide a slow initial rate-of-rise in drug concentrations and a delayed time to the maximum concentration. Symptomology of multiple sclerosis walking impairment, or MS Walking, has been associated with dysregulation of the NMDA receptor/glutamate signaling, as has been reported in Parkinson’s disease. Symptoms, therefore, may be improved by modulating over-activated NMDA receptor/glutamate signaling. These symptoms are present during waking hours, not while the individual is asleep. As a result, an effective treatment should provide relief beginning in the morning, and be sustained throughout the day, while not disrupting sleep.
Walking impairment affects a majority of the approximately 400,000 multiple sclerosis patients in the United States. MS Walking remains an area of high unmet need, even though there is one approved product on the market for the indication. The company's market research suggests that a high proportion of multiple sclerosis patients develop walking impairment, significantly impacting both quality of life and independence. Additionally, physician satisfaction with current treatment options is low, and payers find current treatment to be inappropriate for newly diagnosed patients and effective only in a minority of patients.
The company plan to initiate a Phase 3 study of ADS-5102 for patients with MS Walking early in the second quarter of 2018, based on the feedback the company received from its End-of-Phase 2 meeting with the FDA. The company's Phase 2, 4-week proof-of-concept study showed a significant benefit in walking speed versus placebo on both mean value and the proportion of participants with a clinically significant 17% improvement. The results for timed-up-and-go (TUG) and 2-minute walking test (2MWT) also suggested benefit on other aspects of mobility and walking.
The company's Phase 3 program is planned to consist of two Phase 3 studies, a pivotal efficacy and safety study, and an open label safety study. Adamas Pharmaceuticals is also completing non-clinical studies to support the approval in this multiple sclerosis population. If the first pivotal Phase 3 study is successful, the company intend to meet with the FDA to confirm the filing requirements for this supplemental NDA.
**GOCOVRI (ADS-5102) in Research and Potential Development for Additional Indications **
Adamas Pharmaceuticals is continuing to review the results of preclinical studies, clinical trials, and case reports published in peer reviewed medical journals to evaluate additional potential indications for ADS-5102, including the treatment of wearing OFF and delaying motor complications in Parkinson’s disease, tardive dyskinesia, Huntington’s chorea, Tourette syndrome, and non-motor disorders, including depression, and anti-psychotic induced weight gain. The company expect to select additional indications for ADS-5102 by the first quarter of 2019.
Product Candidates:
ADS-4101 in Development for the Treatment of Partial Onset Seizures in Patients with Epilepsy
ADS-4101 is an investigational high-dose, modified release lacosamide capsule, taken once-daily at bedtime. Lacosamide is an anti-epilepsy active ingredient previously approved by the FDA and currently marketed by UCB SA/NV as VIMPAT ® (lacosamide). Based upon the patents and regulatory exclusivities listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations , also known as the Orange Book, it is estimated that VIMPAT will lose patent exclusivity in 2022. ADS-4101 was designed to temper the initial rate-of-rise in lacosamide concentrations, potentially improving the adverse event profile and dose limitations due to dizziness following administration of VIMPAT.
Epilepsy affects an estimated three million Americans, of which approximately 2/3 have partial onset seizures. Of those people with partial onset seizures, about 30% of patients have poor seizure control with current anti-epilepsy drugs. There are limited data on the temporal distribution of seizures over the 24-hour day; however, published studies suggest that seizures occur in a diurnal pattern, characterized by a peak between 11 a.m. and 5 p.m. and lowest between 11 p.m. and 5 a.m. Thus, by matching the timing pattern of seizures to the concentration of the anti-epileptic drug, with a higher drug concentration during the day and lower drug concentration during the night, ADS-4101 may enable improved seizure control for adults with epilepsy in the United States.
Adamas Pharmaceuticals has completed two Phase 1 studies of ADS-4101 in healthy volunteers. The Phase 1a study showed that a single 400 mg dose of ADS-4101 was better tolerated compared to the equivalent dose of VIMPAT immediate release tablets. The data also demonstrated that ADS-4101 exhibited the desired pharmacokinetic properties, namely a reduced rate of initial rise and delayed time to maximum drug concentration appropriate for bedtime dosing. The recently completed and reported results of a multi-dose Phase 1b study demonstrated that a 600 mg dose of ADS-4101, taken once-nightly, provided a 1.5 to 2.5-fold increase in average lacosamide concentrations throughout the day compared to the maximum approved daily dose of 400 mg, taken as 200 mg twice-daily (BID), of VIMPAT immediate release tablets in healthy volunteers, with comparable tolerability.
The company expect to meet with the FDA in a meeting regarding its planned Phase 3, pivotal program for ADS-4101 in the first half of 2018. The company's proposed clinical development program includes two Phase 3 studies: a robust pivotal study comparing 400 mg and 600 mg of ADS-4101 to placebo, as well as the active comparator, VIMPAT, and an open-label extension study. Subject to the feedback from the FDA, the company anticipate that the Phase 3 study would enroll starting in 2019 and complete enrollment in 2020. The timing of the ADS-4101 clinical development program and its potential approval in the United States is planned to allow it to optimize ADS-4101’s intellectual property protections and market opportunity.
New Product Discovery–Advancing the Product Pipeline
The company continue to apply its “time-dependent biology” approach to identify CNS diseases for which the company can drive significant improvements in efficacy without compromising tolerability. Research programs underway include:
Additional programs in epilepsy, based upon its seizure profile discoveries;New programs in psychiatry;Additional Parkinson’s products, alone and potentially in combination with ADS-5102; andAdditional Multiple sclerosis products, alone and potentially in combination with ADS-5102.
The company anticipate conducting four to five discovery projects per year, with the goal to nominate one additional clinical development program per year.
Partnered Products:
Namzaric ® and Namenda XR ® for the Treatment of Moderate to Severe Dementia of an Alzheimer’s Type
Namzaric (memantine hydrochloride extended release and donepezil hydrochloride) capsules and Namenda XR (memantine hydrochloride) extended release capsules are two commercially available medicines, which are currently marketed by Forest, an indirect wholly-owned subsidiary of Allergan plc, in the United States for the treatment of moderate to severe Alzheimer’s disease. Although Adamas Pharmaceuticals is eligible to receive royalties on net sales of Namenda XR beginning in June 2018, the company do not expect to receive such royalties because of the potential entry of generic versions of Namenda XR. Adamas Pharmaceuticals is eligible to receive royalties on net sales of Namzaric beginning in May of 2020.
Upcoming Milestones
The company expect the following milestones to occur over the next two years:
GOCOVRI TM
Providing updates on its commercial progress with GOCOVRI quarterly;Presenting data at key annual scientific meetings, including the American Academy of Neurology (AAN), Movement Disorder Society (MDS), as well as publishing additional preclinical, Phase 1 and Phase 3 results for GOCOVRI; andReporting final EASE LID 2 Phase 3 open-label safety and efficacy data.
ADS-5102 (GOCOVRI)
Starting first Phase 3 study in MS Walking in early second quarter 2018;Starting an open-label safety and efficacy study by fourth quarter 2018;Completing enrollments in a first Phase 3 study in MS Walking by the second half of 2019; andAdvancing additional indications for ADS-5102 by first quarter 2019.
ADS-4101
Conducting a meeting with the FDA in the first half of 2018; and Enrolling in Phase 3 study patients with partial onset seizures with epilepsy in 2019-2020 (pending FDA feedback).
New Product Development
Advancing two research programs into clinical development by the second half of 2020.
License agreement with Allergan
In November 2012, the company granted Allergan an exclusive license, with right to sublicense, certain of its intellectual property rights relating to human therapeutics containing memantine in the United States. In connection with these rights, Allergan markets and sells Namzaric and Namenda XR for the treatment of moderate to severe dementia related to Alzheimer’s disease. Pursuant to the agreement, Allergan made an upfront payment of $65.0 million. The company earned and received additional cash payments totaling $95.0 million upon achievement by Allergan of certain development and regulatory milestones. Under the agreement, external costs incurred related to the prosecution and litigation of intellectual property rights are reimbursable.
Adamas Pharmaceuticals is entitled to receive royalties on net sales in the United States by Allergan, its affiliates, or any of its sublicensees of controlled-release versions of memantine products covered by the terms of the license agreement. Beginning in May 2020, the company will be entitled to receive royalties in the low to mid-teens from Allergan for sales of Namzaric in the United States. Beginning in June 2018, the company will be entitled to receive royalties in the low to mid-single digits for sales of Namenda XR in the United States. Allergan’s obligation to pay royalties with respect to fixed-dose memantine-donepezil products, including Namzaric, continues until the later of ![]() 15 years after the commercial launch of the first fixed-dose memantine-donepezil product by Allergan in the United States or (ii) the expiration of the Orange Book listed patents for which Allergan obtained rights from it covering such product. However, Allergan’s obligation to pay royalties for any product covered by the license is eliminated in any quarter where there is significant competition from generics. For further information, see Litigation and Other Legal Proceedings in “ Note 8 - Commitments and Contingencies ” in the accompanying “Notes to Consolidated Financial Statements” in this Annual Report. As stated above, the company do not expect to receive royalties on sales of Namenda XR because of the potential entry of generic versions of Namenda XR.
15 years after the commercial launch of the first fixed-dose memantine-donepezil product by Allergan in the United States or (ii) the expiration of the Orange Book listed patents for which Allergan obtained rights from it covering such product. However, Allergan’s obligation to pay royalties for any product covered by the license is eliminated in any quarter where there is significant competition from generics. For further information, see Litigation and Other Legal Proceedings in “ Note 8 - Commitments and Contingencies ” in the accompanying “Notes to Consolidated Financial Statements” in this Annual Report. As stated above, the company do not expect to receive royalties on sales of Namenda XR because of the potential entry of generic versions of Namenda XR.




