Arrowhead Pharmaceuticals
Business
Arrowhead (ARWR) develops medicines that treat intractable diseases by silencing the genes that cause them. Using a broad portfolio of RNA chemistries and efficient modes of delivery, Arrowhead therapies trigger the RNA interference mechanism to induce rapid, deep and durable knockdown of target genes. RNA interference, or RNAi, is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Deemed to be one of the most important recent discoveries in life science with the potential to transform medicine, the discoverers of RNAi were awarded a Nobel Prize in 2006 for their work. Arrowhead’s RNAi-based therapeutics leverage this natural pathway of gene silencing.
In fiscal 2017, Arrowhead refocused its resources on therapeutics that exclusively utilize the company’s Targeted RNAi Molecule (TRiMTM) platform technology. Therapeutics built on the TRiMTM platform have demonstrated high levels of pharmacologic activity in multiple animal models spanning several therapeutic areas. TRiMTM enabled therapeutics offer several potential advantages over prior generation and competing technologies, including: simplified manufacturing and reduced costs; multiple routes of administration including subcutaneous injection and inhaled administration; ability to target multiple tissue types including liver, lung, and tumors; and the potential for improved safety and reduced risk of intracellular buildup, because there are less metabolites from smaller, simpler molecules.
As part of the refocusing of resources, Arrowhead announced in November 2016 that it would be discontinuing all clinical programs, that utilized the intravenously administered dynamic polyconjugate (DPC), or EX1, delivery vehicle. The decision to discontinue development of EX1-containing programs was based primarily on two factors. First, during discussions with regulatory agencies and outside experts, it became apparent that there would be substantial delays in all clinical programs that utilize EX1, while the Company further explored the cause of deaths in a non-clinical toxicology study in non-human primates exploring doses of EX1 higher than those planned to be used in humans. Second, the Company had made substantial advances in RNA chemistry and targeting, resulting in large potency gains for development programs utilizing the TRiMTM technology, making EX1 no longer necessary.
Pipeline Overview
Arrowhead is focused on developing innovative drugs for diseases with a genetic basis, typically characterized by the overproduction of one or more proteins. The depth and versatility of its RNAi technologies enable it to address conditions in virtually any therapeutic area and pursue disease targets that are not otherwise addressable by small molecules and biologics.
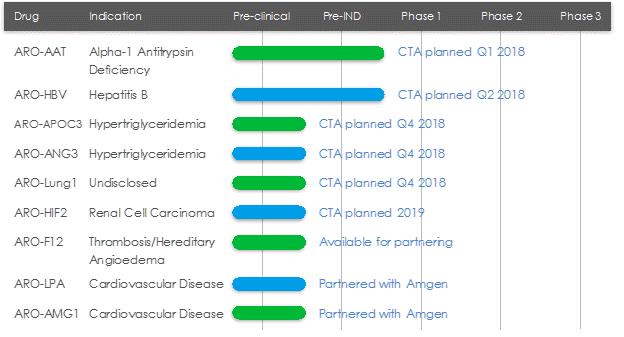
ARO-AAT
ARO-AAT is an RNAi therapeutic candidate for the treatment of liver disease associated with alpha-1 antitrypsin deficiency. ARO-AAT is designed to knock down the Alpha-1 antitrypsin (AAT) gene transcript and reduce the hepatic production of the mutant AAT protein. ARO-AAT is a next-generation subcutaneously administered compound that follows previous generation AAT compound ARC-AAT. A Clinical Trial Application (CTA) is planned for the first quarter of 2018.
Goal of ARO-AAT Treatment
The goal of ARO-AAT treatment is prevention and potential reversal of Z-AAT accumulation-related liver injury and fibrosis. Reduction of inflammatory Z-AAT protein, which has been clearly defined as the cause of progressive liver disease in AATD patients, is important as it is expected to halt the progression of liver disease and allow fibrotic tissue repair.
Alpha-1 Antitrypsin Deficiency (AATD)
AATD is a genetic disorder associated with liver disease in children and adults, and pulmonary disease in adults. AAT is a circulating glycoprotein protease inhibitor that is primarily synthesized and secreted by liver hepatocytes. Its physiologic function is the inhibition of neutrophil proteases to protect healthy tissues during inflammation and prevent tissue damage. The most common disease variant, the Z mutant, has a single amino acid substitution that results in improper folding of the protein. The mutant protein cannot be effectively secreted and accumulates in globules in the hepatocytes. This triggers continuous hepatocyte injury, leading to fibrosis, cirrhosis, and increased risk of hepatocellular carcinoma.
Current Treatments
Individuals with the homozygous PiZZ genotype have severe deficiency of functional AAT leading to pulmonary disease and hepatocyte injury and liver disease. Lung disease is frequently treated with AAT augmentation therapy. However, augmentation therapy does nothing to treat liver disease, and there is no specific therapy for hepatic manifestations. There is a significant unmet need as liver transplant, with its attendant morbidity and mortality, is currently the only available cure.
The Alpha-1 Project
Arrowhead has an agreement with The Alpha-1 Project (TAP), the venture philanthropy subsidiary of the Alpha-1 Foundation. TAP’s mission is to support organizations in pursuit of cures and therapies for lung and liver disease caused by AATD. Under the terms of the agreement, TAP has partially funded development of ARO-AAT. In addition to the funding, TAP will make its scientific advisors available to Arrowhead, assist with patient recruitment for clinical trials with its Alpha-1 Foundation Patient Research Registry, and engage in other collaborative efforts that support development of ARO-AAT.
ARO-HBV
ARO-HBV is an RNAi therapeutic candidate for the treatment of chronic hepatitis B infection with the goal of achieving a functional cure. ARO-HBV is a next-generation subcutaneously administered compound that follows previous generation HBV compounds ARC-520 and ARC-521. A CTA is planned for the second quarter of 2018.
Goal of ARO-HBV Treatment
ARO-HBV is designed to silence the production of all HBV gene products with the goal of achieving a functional cure. The siRNAs target multiple components of HBV production including the pregenomic RNA that would be reverse transcribed to generate the viral DNA. The siRNAs intervene at the mRNA level, upstream of where NUCs act, and target the mRNAs that produce HBsAg proteins, the viral polymerase, the core protein that forms the capsid, the pre-genomic RNA, the HBeAg, and the hepatitis B X antigen (HBxAg). NUCs are effective at reducing production of viral particles, but are ineffective at controlling production of HBsAg and other HBV gene products. Arrowhead believes that a reduction in the production of HBsAg and other proteins that NUCs are ineffective at controlling is necessary to effective HBV therapy, because those proteins are thought to be major contributors to repression of the immune system and the persistence of liver disease secondary to HBV infection.
Chronic Hepatitis B Virus
According to the World Health Organization, 240 million people worldwide are chronically infected with hepatitis B virus, of which 500,000 to 1,000,000 people die each year from HBV-related liver disease. Chronic HBV infection is defined by the presence of hepatitis B surface antigen (HBsAg) for more than six months. In the immune tolerant phase of chronic infection, which can last for many years, the infected person typically produces very high levels of viral DNA and viral antigens. However, the infection is not cytotoxic and the carrier may have no symptoms of illness. Over time, the ongoing production of viral antigens causes inflammation and necrosis, leading to elevation of liver enzymes such as alanine and aspartate transaminases, hepatitis, fibrosis, and liver cancer (hepatocellular carcinoma, or HCC). If untreated, as many as 25% to 40% of chronic HBV carriers ultimately develop cirrhosis or HCC. Antiviral therapy is generally prescribed when liver enzymes become elevated.
The company see the need for a next generation HBV treatment with a finite treatment period and an attractive dosing regimen, and that can be used at earlier stages of disease. The company believe a novel therapeutic approach that can effectively treat or provide a functional cure (seroclearance of HBsAg and with or without development of excess patient antibodies against HBsAg) has the potential to take significant market share and may expand the available market to include patients that are currently untreated.
Current Treatments
The current standard of care for treatment of chronic HBV infection is a daily oral dose of nucleotide/nucleoside analogs (NUCs) or a regimen of interferon injections for approximately one year. NUCs are generally well tolerated, but patients may need lifetime treatment because viral replication often rebounds upon cessation of treatment. Interferon therapeutics can result in a functional cure in 10-20% of some patient types, but treatment is often associated with significant side effects, including severe flu-like symptoms, marrow suppression, and autoimmune disorders.
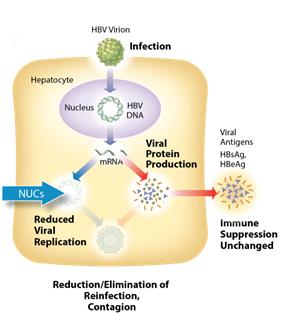 Figure 1: Mechanism of action NUCs
Figure 1: Mechanism of action NUCs
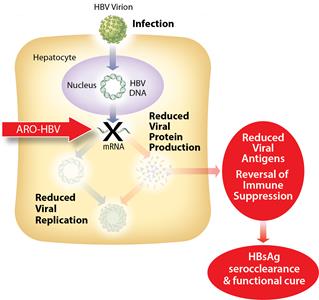 Figure 2: Mechanism of action ARO-HBV
Figure 2: Mechanism of action ARO-HBV
ARO-APOC3
ARO-APOC3 is designed to reduce production of Apolipoprotein C-III (apoC-III), a component of triglyceride rich lipoproteins (TRLs) including VLDL and chylomicrons and a key regulator of triglyceride metabolism. The company believes that knocking down the hepatic production of apoC-III may result in reduced VLDL synthesis and assembly, enhanced breakdown of TRLs, and better clearance of VLDL and chylomicron remnants. A CTA is planned for the fourth quarter of 2018.
Hypertriglyceridemia
Elevated triglyceride levels are an independent risk factor for cardiovascular disease. Severely elevated triglycerides (often over 2,000 mg/dL) in patients with familial chylomicronemia syndrome (FCS), a rare genetic disorder, can result in potentially fatal acute pancreatitis.
ARO-ANG3
ARO-ANG3 is designed to reduce production of angiopoietin-like protein 3 (ANGPTL3), a liver synthesized inhibitor of lipoprotein lipase and endothelial lipase. ANGPTL3 inhibition has been shown to lower serum LDL, serum and liver triglyceride and has genetic validation as a novel target for cardiovascular disease. A CTA is planned for the fourth quarter of 2018.
Hyperlipidemia and Hypertriglyceridemia
Hyperlipidemia and hypertriglyceridemia are risk factors for atherosclerotic coronary heart disease and cardiovascular events.
ARO-LUNG1
ARO-LUNG1 is Arrowhead’s first therapeutic candidate to utilize the TRiMTM platform to target an undisclosed disease of the lung. A CTA is planned for the fourth quarter of 2018.
ARO-HIF2
ARO-HIF2 is being developed for the treatment of clear cell renal cell carcinoma (ccRCC). ARO-HIF2 is designed to inhibit the production of HIF-2α, which has been linked to tumor progression and metastasis in ccRCC. Arrowhead believes it is an attractive target for intervention because over 90% of ccRCC tumors express a mutant form of the Von Hippel-Landau protein that is unable to degrade HIF-2α, leading to its accumulation during tumor hypoxia and promoting tumor growth. It is the first drug candidate to utilize the TRiMTM platform to target tumors. A CTA is planned for 2019.
ARO-F12
ARO-F12 is in development as a potential treatment for factor 12 (F12) mediated diseases such as hereditary angioedema (HAE) and thromboembolic disorders. Factor 12 initiates the intrinsic coagulation pathway and reducing its production using Arrowhead’s RNAi technology may present opportunities in both disease areas.
Thrombosis and HAE
Thrombosis is the formation of blood clots that can obstruct blood flow. Broadly speaking, thrombosis can occur in veins or arteries and may cause serious repercussions if not treated. HAE is a rare genetic disorder with a prevalence of approximately 1/5,000-1/10,000. Patients with HAE can experience recurrent and dangerous acute inflammatory attacks in multiple tissues, with attacks of laryngeal edema being particularly serious and potentially fatal.
Partner-based Programs
ARO-LPA (AMG 890) and ARO-AMG1
ARO-LPA (AMG 890) is designed to reduce production of apolipoprotein A, a key component of lipoprotein(a), which has been genetically linked with increased risk of cardiovascular diseases, independent of cholesterol and LDL levels. Amgen acquired a worldwide, exclusive license in September 2016 to develop and commercialize ARO-LPA (AMG 890).
ARO-AMG1 is being developed against an undisclosed genetically-validated cardiovascular target under a license and collaboration agreement with Amgen.
Under the terms of the agreements taken together for ARO-LPA (AMG 890) and ARO-AMG1, the Company received $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company’s Common Stock, and the Company is eligible to receive up to $617 million in option payments and development, regulatory and sales milestone payments. The Company is further eligible to receive single-digit royalties for sales of products under the ARO-AMG1 agreement and up to low double-digit royalties for sales of products under the ARO-LPA (AMG 890) agreement.
RNA Interference & the Benefits of RNAi Therapeutics
RNA interference (RNAi) is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Deemed to be one of the most important recent discoveries in life science with the potential to transform medicine, the discoverers of RNAi were awarded a Nobel Prize in 2006 for their work. RNAi-based therapeutics may leverage this natural pathway of gene silencing to target and shut down specific disease-causing genes.
Small molecule and antibody drugs have proven effective at inhibiting certain cell surface, intracellular, and extracellular targets. However, other drug targets have proven difficult to inhibit with traditional drug-based and biologic therapeutics. Developing effective drugs for these targets would have the potential to address large underserved markets for the treatment of many diseases. Using the ability to specifically silence any gene, RNAi therapeutics may be able to address previously “undruggable” targets, unlocking the market potential of such targets.
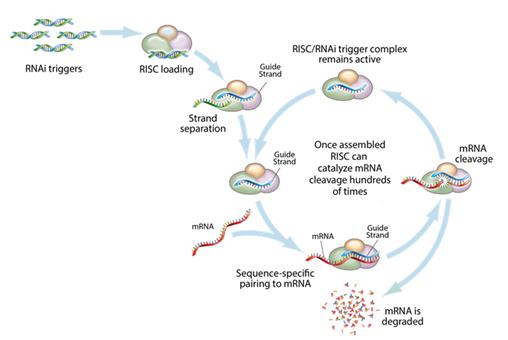
Figure 3: Mechanism of RNA interference
This figure depicts the mechanism by which gene silencing occurs. Double stranded RNAi triggers are introduced into a cell and get loaded into the RNA-induced silencing complex, (RISC). The strands are then separated, leaving an active RISC/RNAi trigger complex. This complex can then pair with and degrade the complementary messenger RNAs (mRNA), and stop the production of the target proteins. RNAi is a catalytic process, so each RNAi trigger can degrade mRNA hundreds of times, which results in a relatively long duration of effect for RNAi therapeutics.
Key Advantages of RNAi as a Therapeutic Modality
- Silences the expression of disease causing genes;
- Potential to address any target in the transcriptome including previously "undruggable" targets;
- Rapid lead identification;
- High specificity;
- Opportunity to use multiple RNA sequences in one drug product for synergistic silencing of related targets; and
- RNAi therapeutics are uniquely suited for personalized medicine through target and cell specific delivery and gene knockdown.
Targeted RNAi Molecule (TRiMTM) Platform
Arrowhead’s Targeted RNAi Molecule (TRiMTM) platform utilizes ligand-mediated delivery and is designed to enable tissue-specific targeting while being structurally simple. Targeting has been core to Arrowhead’s development philosophy and the TRiMTM platform builds on more than a decade of work on actively targeted drug delivery vehicles. Arrowhead scientists have discovered ways to progressively “TRiM” away extraneous features and chemistries and retain optimal pharmacologic activity.
The TRiMTM platform comprises a highly potent RNA trigger identified using Arrowhead’s proprietary trigger selection rules and algorithms with the following components optimized, as needed, for each drug candidate: a high affinity targeting ligand; various linker and chemistries; structures that enhance pharmacokinetics; and highly potent RNAi triggers with sequence specific stabilization chemistries.
Therapeutics developed with the TRiMTM platform offer several advantages: simplified manufacturing and reduced costs; multiple routes of administration; and potential for improved safety because there are less metabolites from smaller molecules, thereby reducing the risk of intracellular buildup. At Arrowhead, the company also believe that for RNAi to reach its true potential, it must target organs outside the liver. Arrowhead is leading this expansion with the TRiMTM platform, which has the potential to reach multiple tissues, including liver, lung, and tumor.
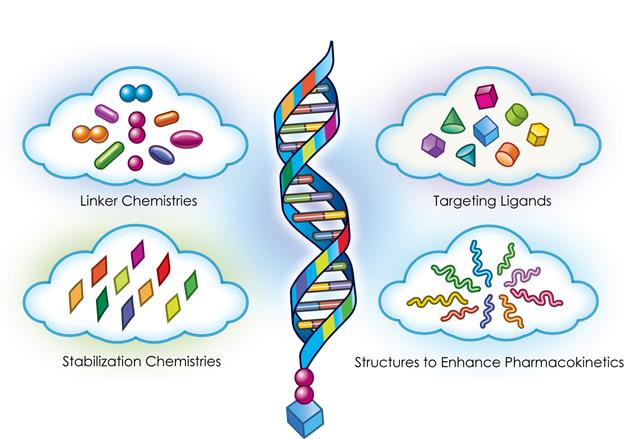 Figure 4: Targeted RNAi Molecule (TRiMTM) Schematic
Figure 4: Targeted RNAi Molecule (TRiMTM) Schematic
RNA Chemistries
The structure and chemistries of the oligonucleotide molecules used to trigger the RNAi mechanism can be tailored for optimal activity. Arrowhead’s broad portfolio of RNA trigger structures and chemistries, including some proprietary structures, enable the company to optimize each drug candidate on a target-by-target basis and utilize the combination of structure and chemical modifications that yield the most potent RNAi trigger.
As a component of the TRiMTM platform, Arrowhead’s design philosophy for RNA chemical modifications is to start with a structurally simple molecule and add only selective modification and stabilization chemistries as necessary to achieve the desired level of target knockdown and duration of effect. The conceptual framework for the stabilization strategy starts with a more sophisticated RNAi trigger screening and selection process that identifies potent sequences rapidly in locations that others may miss. The company pursue chemical stabilization strategies with a target duration of effect of 30-90 days and typically limit the use of strategies that produce longer activity because the company anticipate that such strategies will increase long-term safety risks.
Intellectual Property and Key Agreements
The Company controls approximately 333 issued patents (including 179 directed to RNAi trigger molecules; 17 directed to targeting groups or targeting compounds; and 19 for hydrodynamic gene delivery), including European validations, and approximately 280 currently pending patent applications worldwide from 53 different patent families. The Company’s patent applications have been filed throughout the world, including, in the United States, Argentina, ARIPO (Africa Regional Intellectual Property Organization), Australia, Brazil, Canada, Chile, China, Eurasian Patent Organization, Europe, GCC (Gulf Cooperation Council), Hong Kong, Israel, India, Indonesia, Iraq, Jordan, Japan, Republic of Korea, Lebanon, Mexico, New Zealand, OAPI (African Intellectual Property Organization), Peru, Philippines, Russian Federation, Saudi Arabia, Singapore, Thailand, Taiwan, Uruguay, Venezuela, Vietnam, and South Africa.
RNAi Triggers
The Company owns or has filed patent applications directed to RNAi trigger molecules, which serve as the foundation of Arrowhead’s TRiMTM platform, and are targeted to reduce expression of several gene targets, including the following:
| Patent Group | Estimated Year(s) of Expiration |
|---|---|
| RNAi Triggers Gene Target | |
| HBV | 2032, 2036, 2037 |
| AAT | 2035 |
| LPA | 2036 |
| Factor 12 | 2036 |
| HIF2a | 2034, 2036 |
| RRM2 | 2031 |
| APOC3 | 2035 |
| a-ENaC | 2028 |
| ß-ENaC | 2031 |
| ß-Catenin | 2033 |
| Cx43 | 2029 |
| HCV | 2023 |
| HIF1A | 2026 |
| HRH1 | 2027 |
| HSF1 | 2030, 2032 |
| FRP-1 | 2026 |
| KRAS | 2033 |
| PDtype4 | 2026 |
| PI4Kinase | 2028 |
| SYK | 2027 |
| TNF-a | 2027, 2028 |
Delivery Technologies
The delivery technology-related patent applications, which include components used in Arrowhead’s TRiMTM platform, have been filed and issued in the United States, Australia, Canada, Europe, France, Germany, Italy, Spain, Switzerland, United Kingdom, India, Japan, Mexico, New Zealand, Philippines, Russia, South Korea, Singapore, and South Africa. The Company also controls a number of patents directed to hydrodynamic nucleic acid delivery, which issued in the United States, Australia and Europe (validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, the United Kingdom, Hungary, Ireland, Italy, Netherlands and Sweden). The approximate year of expiration for each of these various groups of patents are set forth below:
| Patent Group | Estimated Year of Expiration |
|---|---|
| Targeting ligands and other RNAi delivery technologies | |
| Targeting groups (Galactose derivative trimer-PK) | 2031 |
| Targeting groups (avß3 integrin) | 2035 |
| Targeting groups (avß6 integrin) | 2037 |
| Targeting groups (Galactose derivative ligands) | 2037 |
| RNAi agent design (5'-phosphate mimic) | 2037 |
| Physiologically labile linkers | 2036 |
| Biologically cleavable linkers | 2036 |
| Transferrin targeting | 2028 |
| LDLR targeting | 2028 |
| Peptide targeting (CPP-Arg) | 2028 |
| Peptide targeting (YM3-10H) | 2032 |
| . | |
| Hydrodynamic delivery | |
| Second iteration | 2020 |
| Third iteration | 2024 |
The RNAi and drug delivery patent landscapes are complex and rapidly evolving. As such, the company may need to obtain additional patent licenses prior to commercialization of its candidates.
Non-Exclusively Licensed Patent Rights obtained from Roche
On October 21, 2011, Arrowhead acquired the RNAi therapeutics business of Hoffmann-La Roche, Inc. and F. Hoffmann-La Roche Ltd. (collectively, “Roche”). Roche built this business unit in a manner that only a large pharmaceutical company is capable of: backed by expansive capital resources, Roche systematically acquired technologies, licensed expansive intellectual property rights, attracted leading scientists, and developed new technologies internally. At a time when the markets were questioning whether RNAi could become a viable therapeutic modality, the company saw great promise in the technology broadly and the quality of what Roche built specifically. The acquisition provided it with two primary sources of value:
- Broad freedom to operate with respect to key patents directed to the primary RNAi-trigger formats: canonical, UNA, meroduplex, and dicer substrate structures; and
- A large team of scientists experienced in RNAi and oligonucleotide delivery.
Pursuant to this acquisition, Roche assigned to Arrowhead its entire rights under certain licenses including: the License and Collaboration Agreement between Roche and Alnylam Pharmaceuticals, Inc. (“Alnylam”) dated July 8, 2007 (the “Alnylam License”); the Non-Exclusive Patent License Agreement between Roche and MDRNA, Inc. dated February 12, 2009 (“MDRNA License”); and the Non-Exclusive License Agreement between Roche and City of Hope dated September 19, 2011 (the “COH License”) (Collectively the “RNAi Licenses”). The RNAi Licenses provide the Company with non-exclusive, worldwide, perpetual, irrevocable, royalty-bearing rights and the right to sublicense a broad portfolio of intellectual property relating to the discovery, development, manufacture, characterization, and use of therapeutic products that function through the mechanism of RNA interference for specified targets.
Terms of the 2007 Alnylam License
The Alnylam License provides it with a non-exclusive, worldwide, perpetual, irrevocable, royalty-bearing right and sublicensable license under Alnylam’s rights in certain intellectual property existing as of its effective date, to engage in discovery, development, commercialization and manufacturing activities, including to make, have made, use, offer for sale, sell and import certain licensed products in certain fields. The fields include the treatment or prophylaxis of indications comprising an RNAi compound complementary to, and function in mediating the RNAi of, a target known or believed to be primarily implicated in one or more primary therapeutic areas. The primary therapeutic areas are cancer, hepatic, metabolic disease and pulmonary disease. The hepatic therapeutic area specifically excludes targets of infectious pathogen.
The Alnylam License excludes access to intellectual property specifically related to “Blocked Targets.” “Blocked Targets” are those targets that are subject to a contractual obligation of a pre-existing agreement between Alnylam and its alliance partners.
Under the Alnylam License, the company may be obligated to pay development and sales milestone payments of up to the mid to upper double-digit millions of dollars for each licensed product that progresses through clinical trials in a particular indication, receives marketing approval for that indication and is the subject of a first commercial sale. Additionally, the company may be obligated to pay mid to high single-digit percentage royalties on sales of such products.
Core Patents relating to RNAi
The RNAi Licenses include patents relating to the general structure, architecture, and design of double-stranded oligonucleotide molecules, which engage RNA interference mechanisms in a cell. These rights include the “Tuschl II” patents, including issued U.S. Patent Nos. 7,056,704; 7,078,196; 7,078,196; 8,329,463; 8,362,231; 8,372,968; and 8,445,327; “Tuschl I” patents, including U.S. Patent Nos. 8,394,628 and 8,420,391; and allowed “Tuschl I” patent application, U.S. Publication No. 2011024446; “City of Hope” patents, including U.S. Patent No. 8,084,599; and “Kreutzer-Limmer” patents assigned to Alnylam, including U.S. Patent Nos. 7,829,693; 8,101,594; 8,119,608; 8,202,980; and 8,168,776.
Thomas Tuschl is the first named inventor on “Tuschl I” and “Tuschl II.” “Tuschl I” patents refers to the patents arising from the patent application entitled “The Uses of 21-23 Sequence-Specific Mediators of Double-Stranded RNA Interference as a Tool to Study Gene Function and as a Gene-Specific Therapeutic.” “Tuschl II” patents refer to the patents and patent applications arising from the patent application entitled “RNA Interference Mediating Small RNA Molecules.” “City of Hope” is the first named assignee of certain core RNAi trigger patents. The second named assignee of these patents is Integrated DNA Technologies, Inc. Kreutzer-Limmer patents refer to the Alnylam patents and patent applications, relating to core siRNA IP, which includes inventors Roland Kreutzer and Stefan Limmer.
Chemical modifications of double-stranded oligonucleotides
The RNAi Licenses also include patents related to modifications of double-stranded oligonucleotides, including modifications to the base, sugar, or internucleoside linkage, nucleotide mimetics, and end modifications, which do not abolish the RNAi activity of the double-stranded oligonucleotides. Also included are patents relating to modified double-stranded oligonucleotides, such as meroduplexes described in U.S. Patent No. 9,074,205 assigned to Marina Biotech (f/k/a MDRNA, Inc.), and microRNAs described in U.S. Patent Nos. 7,582,744; 7,674,778, and 7,772,387 assigned to Alnylam, as well as U.S. Patent No. 8,314,227 related to unlocked nucleic acids (UNA). The ‘227 patent was assigned by Marina Biotech to Arcturus Therapeutics, Inc. but remains part of the MDRNA License. The RNAi Licenses also include rights from INEX/Tekmira relating to lipid-nucleic acid particles, and oligonucleotide modifications to improve pharmacokinetic activity including resistance to degradation, increased stability, and more specific targeting of cells from Alnylam and Ionis Pharmaceuticals, Inc. (f/k/a ISIS Pharmaceuticals, Inc.).
Manufacturing techniques for the double-stranded oligonucleotide molecules or chemical modifications
The RNAi Licenses also include patents relating to the synthesis and manufacture of double-stranded oligonucleotide molecules for use in RNA interference, as well as chemical modifications of such molecules, as described above. These include methods of synthesizing the double-stranded oligonucleotide molecules such as in the core “Tuschl I” allowed U.S. Application No. 12/897,749, the core “Tuschl II” U.S. Patent Nos. 7,056,704; 7,078,196; and 8,445,327; and Alnylam’s U.S. Patent No. 8,168,776, as well as methods of making chemical modifications of the double-stranded oligonucleotides such as described in Alnylam’s U.S. Patent No. 7,723,509. Patent applications are currently pending that further cover manufacturing techniques for double-stranded oligonucleotide molecules or chemical modifications.
Uses and Applications of Double-Stranded Oligonucleotide Molecules or Chemical modifications
The RNAi Licenses also include patents related to uses of the double-stranded oligonucleotides that function through the mechanism of RNA interference. These include for example, the core “Tuschl I” U.S. Patent No. 8,394,628 and “Tuschl II” U.S. Patent No. 8,329,463; Alnylam’s U.S. Patent Nos. 7,763,590; 8,101,594, and 8,119,608, and City of Hope‘s U.S. Patent No. 8,084,599. Other more specific uses have been acquired and patent applications are currently pending that cover additional end uses and applications of double-stranded oligonucleotides functioning through RNA interference.
2012 License to Alnylam
In consideration for licenses obtained from Alnylam to certain RNAi intellectual property, in January 2012 the company granted Alnylam a worldwide non-exclusive, sublicensable royalty-bearing license under its broad and target-specific DPC intellectual property rights to research, develop and commercialize RNAi-based products against a single undisclosed target in combination with DPC technology. Under the license to Alnylam, Alnylam may be obligated to pay it development and sales milestone payments of up to the low double-digit millions of dollars for each licensed product that progresses through clinical trials, receives marketing approval and is the subject of a first commercial sale. Additionally, Alnylam may be obligated to pay it low single-digit percentage royalties on sales of such products.
Acquisition of Assets from Novartis
On March 3, 2015, the Company entered into an Asset Purchase and Exclusive License Agreement (the “RNAi Purchase Agreement”) with Novartis pursuant to which the Company acquired Novartis’ RNAi assets and rights thereunder. Pursuant to the RNAi Purchase Agreement, the Company acquired or licensed certain patents and patent applications owned or controlled by Novartis related to RNAi therapeutics, assignment of Novartis’s rights under a license from Alnylam (the “Alnylam-Novartis License”), rights to three pre-clinical RNAi candidates, and a license to certain Novartis assets (the “Licensed Novartis Assets”). The patents acquired from Novartis include multiple patent families covering delivery technologies and RNAi-trigger design rules and modifications. The Licensed Novartis Assets include an exclusive, worldwide right and license, solely in the RNAi field, with the right to grant sublicenses through multiple tiers under or with respect to certain patent rights and know how relating to delivery technologies and RNAi-trigger design rules and modifications. Under the assigned Alnylam-Novartis License, the Company acquired a worldwide, royalty-bearing, exclusive license with limited sublicensing rights to existing and future Alnylam intellectual property (including intellectual property that came under Alnylam’s control on or before March 31, 2016), excluding intellectual property concerning delivery technology, to research, develop and commercialize 30 undisclosed gene targets.
The company see the Roche and Novartis acquisitions as a powerful combination of intellectual property, R&D infrastructure, and RNAi experts. This foundation and substantial progress made by Arrowhead scientists over the last few years enable it to develop what the company think are optimal RNAi therapeutics.
Cardiovascular Collaboration and License Agreements with Amgen
On September 28, 2016, the Company entered into two Collaboration and License agreements and a Common Stock Purchase Agreement with Amgen. Under the First Collaboration and License Agreement, Amgen received an option to a worldwide, exclusive license to ARO-AMG1, an RNAi therapy for an undisclosed genetically validated cardiovascular target. Under the Second Collaboration and License, Amgen received a worldwide, exclusive license to Arrowhead’s novel, RNAi ARO-LPA (AMG 890) program. The ARO-LPA (AMG-890) RNAi molecules are designed to reduce elevated lipoprotein(a), which is a genetically validated, independent risk factor for atherosclerotic cardiovascular disease. In both agreements, Amgen is wholly responsible for clinical development and commercialization. Under the terms of the agreements taken together, the Company has received $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company’s Common Stock, and may receive up to $617 million in option payments and development, regulatory and sales milestone payments. The Company is further eligible to receive single-digit royalties for sales of products under the ARO-AMG1 agreement and up to low double-digit royalties for sales of products under the ARO-LPA (AMG 890) agreement.
Research and Development Facility
Arrowhead’s research and development operations are located in Madison, Wisconsin. Substantially all of the Company’s assets are located either in this facility or in its corporate headquarters in Pasadena. A summary of its research and development resources is provided below:
- Approximately 70 scientists currently;
- State-of-the-art laboratories consisting of 60,000 total sq. ft.;
- Complete small animal facility;
- Primate colony housed at the Wisconsin National Primate Research Center, an affiliate of the University of Wisconsin;
- In-house histopathology capabilities;
- Animal models for cardio metabolic, viral, lung, and oncologic diseases;
- Animal efficacy and safety assessment;
- In-house drug manufacturing capabilities to produce first-in-human GMP (phase appropriate) material;
- Polymer, peptide, oligonucleotide and small molecule synthesis and analytics capabilities (HPLC, NMR, MS, etc.);
- Polymer, peptide and oligonucleotide PK, biodistribution, clearance methodologies; and
- Conventional and confocal microscopy, flow cytometry, Luminex platform, qRT-PCR, clinical chemistry analytics.
Research and Development Expenses
Research and development (R&D) expenses consist of costs incurred in discovering, developing and testing its clinical and preclinical candidates and platform technologies. R&D expenses also include costs related to clinical trials, including costs of contract research organizations to recruit patients and manage clinical trials. Other costs associated with clinical trials include manufacturing of clinical supplies, as well as good laboratory practice (“GLP”) toxicology studies necessary to support clinical trials, both of which are outsourced to cGMP-compliant manufacturers and GLP-compliant laboratories. Total research and development expense for fiscal 2017 was $31.7 million, a decrease from $41.5 million in 2016 and a decrease from $47.3 million in 2015.
At September 30, 2017, the company employed approximately 76 employees in an R&D function, primarily working from its facility in Madison, Wisconsin. These employees are engaged in various areas of research on Arrowhead candidate and platform development including synthesis and analytics, PK/biodistribution, formulation, CMC and analytics, tumor and extra-hepatic targeting, bioassays, live animal research, toxicology/histopathology, clinical and regulatory operations, and other areas. Salaries and payroll-related expenses for its R&D activities were $11.7 million in fiscal 2017, $13.9 million in fiscal 2016, and $11.6 million in fiscal 2015. Laboratory supplies including animal-related costs for in-vivo studies were $7.7 million, $4.3 million, and $3.1 million in fiscal 2017, 2016, and 2015, respectively.
Costs related to the manufacture of clinical supplies, GLP toxicology studies and other outsourced lab studies, as well as clinical trial costs were $21.3 million, $32.6 million, and $41.8 million in fiscal 2017, 2016, and 2015, respectively.
Facility-related costs, primarily rental costs for its leased laboratory in Madison, Wisconsin were $2.3 million, $1.3 million, and $1.0 million in fiscal 2017, 2016, and 2015, respectively. Other research and development expenses were $0.3 million, $3.2 million, and $1.4 million in fiscal 2017, 2016 and 2015, respectively. These expenses are primarily related to milestone payments, which can vary from period to period depending on the nature of its various license agreements, and the timing of reaching various development milestones requiring payment.




