DelMar Pharmaceuticals
Background
DelMar Pharmaceuticals, Inc. (DMPI) is a clinical stage drug development company with a focus on the treatment of cancer. The company's mission is to benefit patients and create shareholder value by developing and commercializing anti-cancer therapies for patients whose tumors exhibit features that make them resistant to, or unlikely to respond to, currently available therapies, particularly for orphan cancer indications where patients have failed, or are unlikely to respond to, currently available therapy.
DelMar Pharmaceuticals is developing VAL-083, a novel, DNA-targeting agent, for the treatment of glioblastoma multiforme (“GBM”) and potentially other solid tumors, including ovarian cancer. VAL-083 is a first-in-class, DNA-targeting chemotherapeutic that demonstrated activity against a range of tumor types in prior Phase 1 and Phase 2 clinical studies sponsored by the US National Cancer Institute (“NCI”). The company's recent research has highlighted the opportunities afforded by VAL-083’s unique mechanism of action and its potential to address unmet medical needs by focusing its development efforts on patients whose tumors exhibit biological features that make them resistant to, or unlikely to respond to, currently available therapies. For example, its research demonstrating VAL-083’s activity in GBM independent of the O6-methyl guanine methyltransferase (“MGMT”) methylation status allows it to focus patient selection based on this important biomarker.
DelMar Pharmaceuticals has initiated two open-label, bio-marker driven Phase 2 studies in MGMT-unmethylated GBM. MGMT is a DNA-repair enzyme that is associated with resistance to temozolomide, the current standard-of-care chemotherapy used in the treatment of GBM. Approximately two out of three GBM patients have MGMT-unmethylated tumors and exhibit a high expression of MGMT, which is correlated with temozolomide treatment failure and poor patient outcomes. The company's research demonstrates that VAL-083’s anti-tumor activity is independent of MGMT expression. In its Phase 2 studies DelMar Pharmaceuticals is using MGMT as a biomarker to identify patients for treatment with VAL-083. If successful, the result of these studies could position VAL-083 for advancement to pivotal clinical studies as a potential replacement for temozolomide in MGMT-unmethylated GBM. The company anticipate presenting interim data from these studies at peer reviewed scientific meetings during calendar 2018 and 2019.
DelMar Pharmaceuticals has received notice to proceed from the US Food and Drug Administration (“FDA”) for a phase 1/2, open-label, multicenter study of VAL-083 in patients with Recurrent Platinum Resistant Ovarian Cancer (“REPROVe”). Platinum-based chemotherapy is standard-of-care in the treatment of ovarian cancer. Nearly all ovarian cancer patients eventually become resistant to platinum (“Pt”) -based chemotherapy leading to treatment failure and poor patient outcomes. DelMar Pharmaceuticals has demonstrated that VAL-083 is active against Pt-resistant ovarian cancer in vitro. Based on ongoing evaluation and recent input from its recently-formed clinical advisory board, DelMar Pharmaceuticals is reassessing the ovarian cancer program. DelMar Pharmaceuticals is in the process of evaluating the best path forward in ovarian cancer and are looking at various strategic options including combination with PARP inhibitors.
In addition to its clinical development activities in the United States, pursuant to its collaboration with Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. (“Guangxi Wuzhou Pharmaceutical Company”), DelMar Pharmaceuticals has provided Guangxi Wuzhou Pharmaceutical Company certain commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. Guangxi Wuzhou Pharmaceutical Company is the only manufacturer presently licensed by the China Food and Drug Administration (“CFDA”) to produce the product for the China market.
DelMar Pharmaceuticals has a broad patent portfolio to protect its intellectual property. The company's patent applications claim composition of matter and methods of use of VAL-083 and related compounds, synthetic methods, and quality controls for the manufacturing process of VAL-083. The company believe that its portfolio of intellectual property rights provides a defensible market position for the commercialization of VAL-083. In addition, VAL-083 has been granted protection under the Orphan Drug Act by the FDA and the European Medicines Agency (“EMA”) for the treatment of gliomas, including GBM. The FDA has also granted Orphan Drug protection to VAL-083 for the treatment of medulloblastoma and ovarian cancer.
The company's corporate development strategy is to advance its drug candidate into multiple clinical studies and then to consider licensing, or acquiring additional product candidates, in order to establish a product pipeline and position it for long-term sustainability and growth of shareholder value. The company believe the experience of its clinical development team will position it to efficiently develop drug candidates that the company may acquire, or license, in the future.
The company intend to continue to evaluate options for its strategic direction. These options may include raising additional capital, the acquisition of another company and / or complementary assets, its sale, or another type of strategic partnership.
Recent Highlights
In September 2018, the company announced that the company had engaged Oppenheimer & Co. Inc. as its strategic advisor to help manage the exploration and evaluation of a wide range of strategic opportunities.As of September 21, 2018, DelMar Pharmaceuticals has enrolled forty of the planned 48 patients in its Phase 2, open-label clinical study of VAL-083 in bevacizumab (Avastin®)-naïve, recurrent glioblastoma multiforme (“rGBM”) patients with MGMT-unmethylated status. This study is being conducted at the MD Anderson Cancer Center (“MDACC”) in Houston, Texas. The study is designed to determine the impact of VAL-083 treatment on overall survival compared to historical reference control.As of September 21, 2018, DelMar Pharmaceuticals has enrolled nine of the planned up to 30 patients in its Phase 2, open-label clinical study of VAL-083 in newly-diagnosed, MGMT-unmethylated, GBM patients being conducted in Guangzhou, China. This study is a single-site study being conducted at Sun Yat-sen University Cancer Center (“SYSUCC”) on newly diagnosed MGMT-unmethylated GBM patients. Patients in this study are being treated with VAL-083 in combination with radiotherapy as a potential alternative to the current standard-of-care chemo-radiation regimen. This study was initiated in September 2017 and is being conducted under the terms of its collaboration with Guangxi Wuzhou Pharmaceutical Company.In June 2018, the company strengthened its Board of Directors and corporate governance with the addition of world-renowned molecular biologist Dr. Napoleone Ferrara and the appointment of Robert E. Hoffman as independent chairman.In May 2018, Saiid Zarrabian was appointed as full-time president and CEO. Mr. Zarrabian had been serving as a director since July 7, 2017, as its interim CEO since November 3, 2017 and as interim president since January 1, 2018.In April 2018, at the American Association for Cancer Research (“AACR”) Annual meeting, the company presented promising research results supporting the potential of VAL-083 in the treatment of cancers for patients whose tumors exhibit features that make them resistant to, or unlikely to respond to, currently available therapies as follows:The company presented incremental VAL-083 preclinical data demonstrating that the combination of VAL-083 and PARP inhibitors may be an effective therapeutic approach for the treatment of cancer. The data show that VAL-083 can synergize PARP inhibitors in both a BRCA–proficient and –deficient setting. Multiple PARP inhibitors are currently approved for the treatment of recurrent breast and ovarian cancer; andThe company presented preclinical data demonstrating that VAL-083 may be beneficial, either as a single-agent, or as part of combination therapy regimens, for difficult-to-treat, or resistant, pediatric high-grade gliomas, including diffuse intrinsic pontine glioma ("DIPG"). DIPG is a rare, inoperable childhood brain tumor with very poor prognosis and a bleak survival outlook. The data shows VAL-083 is active as a single-agent and synergistic with AZD1775, a Wee1 inhibitor, against DIPG cell lines with varying genetic profiles, including p53 and H3.3/H3.1 K27M mutations.In December 2017, the FDA granted Fast Track designation for VAL-083 in rGBM. Fast track designation is designed to expedite the review of drugs that show promise in treating life-threatening diseases and address unmet medical needs, with the goal of getting new treatments to patients earlier. Fast Track designation provides sponsors with an opportunity for increased frequency of communication with FDA to ensure an optimal development plan and to collect appropriate data needed to support drug approval.
VAL-083 Overview
The company's product candidate, VAL-083, a DNA-targeting agent, is a first-in-class, small molecule, chemotherapeutic. “First-in-class” means that VAL-083 embodies a unique molecular structure which is not an analogue or derivative of any approved product, or product under development, for the treatment of cancer. Prior VAL-083 clinical studies supported by the NCI demonstrated activity against a range of cancers including lung, brain, cervical, ovarian tumors, and leukemia. As part of its business strategy, the company leverage and build upon these prior NCI investments and data from more than 40 NCI- Phase 1 and Phase 2 clinical studies with its own research to identify and target unmet medical needs in modern cancer care.
DNA-targeting agents are among the most successful and widely used treatments for cancer. Their efficacy is based on the ability to bind with cancer cell’s DNA and interfere with the process of protein production required for growth and survival of cancer cells.
The company's research demonstrates the mechanism of action of VAL-083 is distinct from other DNA-targeting agents. VAL-083’s anti-cancer activity results from its forming DNA-cross links at the N7 position of guanine leading to DNA double strand breaks, cell-cycle arrest, and cancer cell death. DelMar Pharmaceuticals has presented research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies. These data, combined with clinical activity demonstrated against various cancers in prior NCI-sponsored clinical studies enhance its confidence that VAL-083 may offer an opportunity as a new therapeutic option for patients whose tumors exhibit biological features that cause them to be resistant, or unlikely to respond to, currently available treatments.
DelMar Pharmaceuticals is currently studying VAL-083 in multiple clinical studies for the treatment of GBM, the most common and aggressive form of brain cancer. DelMar Pharmaceuticals has also received clearance from the FDA of an IND for the use of VAL-083 in the treatment of platinum-resistant ovarian cancer.
The FDA Office of Orphan Products Development has granted orphan drug designations to VAL-083 for the treatment of glioma, ovarian cancer and medulloblastoma. VAL-083 has also been granted an orphan drug designation for the treatment of glioma in Europe. Orphan diseases are defined in the United States under the Orphan Drug Act of 1983 as “any disease or condition that affects fewer than 200,000 persons in the United States”. The Orphan Drug Act of 1983 provides financial and other incentives including a seven-year period of market exclusivity in the United States to encourage the development of new treatments for orphan diseases.
VAL-083 Clinical Studies
DelMar Pharmaceuticals is currently developing VAL-083, a novel DNA-targeting agent for the treatment of GBM and potentially other solid tumors, including ovarian cancer. The company's recent research has highlighted the opportunities afforded by VAL-083’s unique mechanism of action and its potential to address unmet medical needs by focusing its development efforts on patients whose tumors exhibit biological features that make them resistant to, or unlikely to respond to, currently available therapies. For example, its research demonstrating VAL-083’s activity in GBM is independent of the MGMT methylation status allows it to focus patient selection based on this important biomarker.
The evaluation of MGMT promotor methylation status has increasingly become common practice in the diagnostic assessment of GBM. In September 2017, the National Comprehensive Cancer Network (“NCCN”), updated guidelines for the standard treatment of GBM based on MGMT methylation status. The company believe these recently published guidelines provide for enhanced opportunities for it to capitalize on VAL-083’s unique mechanism of action by utilizing MGMT methylation as a biomarker to optimize patient selection for its novel DNA-targeting agent to target the majority of GBM patients who are diagnosed with MGMT-unmethylated tumors.
The company's current priority is to leverage this research and VAL-083’s unique mechanism of action to efficiently advance its drug candidate for the most promising indications, including:
MGMT-unmethylated GBM, currently comprising two ongoing separate Phase 2 clinical studies for:
rGBM patients (ongoing study at MDACC); andNewly diagnosed GBM patients (ongoing study at Sun Yat-sen University); and
Potentially platinum-resistant ovarian cancer.
With respect to its STAR-3, Phase 3 study, other than to support the sole remaining enrolled patient, DelMar Pharmaceuticals has finalized the decision to discontinue this clinical study.
MGMT-unmethylated GBM
GBM is the most common and the most lethal form of glioma. According to the Central Brain Tumor Registry of the United States, GBM occurs with an incidence of 3.20 per 100,000 person-years. Approximately 13,000 new cases of GBM were diagnosed in the United States and 16,000 in Europe during 2017. Within the GBM patient population, approximately two-thirds of patients are unmethylated with respect to their MGMT status.
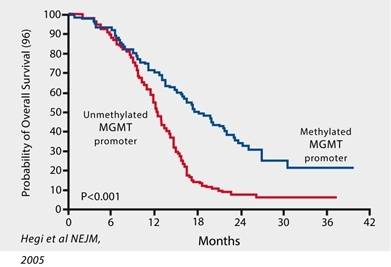
Measurement of MGMT (O6-methyl guanine methyltransferase) methylation status has become routine in clinical practice as a biomarker that correlates with resistance to the standard-of-care chemotherapy with temozolomide (Temodar® “TMZ”), and patient outcomes in GBM. Approximately two-thirds of GBM patients’ tumors are characterized as “MGMT-unmethylated” and exhibit a high expression of MGMT, a naturally occurring DNA-repair enzyme, the activity of which nullifies the chemotherapeutic activity of TMZ. The development of new therapies for MGMT-unmethylated GBM is a significant unmet medical need. Importantly, the most recent update to NCCN guidelines states that the treatment benefit of TMZ is likely to be lower in GBM patients with an unmethylated MGMT promoter, and therefore, allows for withholding of TMZ in the treatment of newly diagnosed GBM patients with MGMT-unmethylated tumors due to lack of efficacy.
DelMar Pharmaceuticals has demonstrated that VAL-083’s anti-tumor mechanism is active independent from the MGMT status in vitro. The company believe this suggests the potential of VAL-083 as a replacement for the current standard-of-care chemotherapy, temozolomide, in MGMT-unmethylated GBM. DelMar Pharmaceuticals is therefore utilizing MGMT-methylation status to identify GBM patients who are unlikely to respond to temozolomide and instead treat them with VAL-083.
The company believe that its research, in the context of the recent amendment to NCCN guidelines, highlights this unmet need and the opportunity for VAL-083 as a potential new standard-of-care in the treatment of MGMT-unmethylated GBM.
Phase 2 Study in MGMT-unmethylated rGBM in Collaboration with University of Texas MD Anderson Cancer Center
In February 2017, the company initiated a biomarker driven, open-label, single-arm Phase 2 study in collaboration with MDACC. This study will enroll up to 48 MGMT-unmethylated GBM patients whose tumors have recurred following treatment with temozolomide. These patients will not have been treated previously with Avastin. The primary endpoint of the study is overall survival. Safety data from this study will become part of the overall safety dossier to support future filings with the FDA and other regulatory agencies.
As of September 21, 2018, forty patients had been enrolled in this Phase 2 study. The original starting dose of 40mg/m2 of VAL-083 on days 1, 2 and 3, of a 21-day cycle, which was based on the results from its previous Phase 1/2 safety study of VAL-083 in patients with recurrent glioma (clinicaltrials.gov identifier: NCT01478178), has continued to demonstrate myelosuppression as the principal side effect of VAL-083, as per prior clinical experience. The safety profile has been well within the existing safety monitoring guidelines described in the present study protocol. However, in consultation with the principal investigator at MDACC, DelMar Pharmaceuticals is amending the protocol for this clinical study to modify the starting dose of VAL-083 to 30mg/m2 on days 1, 2 and 3, of a 21-day cycle for this specific population previously treated with temozolomide. This modification may improve tolerance in this patient population and maximize overall exposure to VAL-083 thereby increasing the number of cycles of drug patients are able to receive. DelMar Pharmaceuticals is also modifying the patient screening platelet count, from 100,000/µL to 125,000/µL, for the same reasons.
It is important for this GBM patient population, which has been heavily pre-treated with temozolomide, to be able to be treated with multiple cycles of VAL-083 without significant hematological toxicities. The company believe the modified dose of VAL-083, in addition to the change in patient eligibility platelet counts, should help provide for enhanced patient safety. The company believe a positive outcome from this study can establish a position for VAL-083 in the treatment of MGMT-unmethylated rGBM.
Based on increased enrollment rates, DelMar Pharmaceuticals is forecasting full enrollment by December 2018. Data from the study will be used to help develop potential future clinical study designs with VAL-083 in MGMT-unmethylated rGBM. The company anticipate providing updates regarding the progress of this open-label study, including safety data and observations regarding outcomes, at scientific meetings during 2018 and 2019. A detailed description of this study can be found at clinicaltrials.gov, Identifier Number: NCT02717962.
Phase 2 Study in Newly Diagnosed MGMT-unmethylated GBM
In September 2017, the company initiated a single arm, biomarker driven, open-label Phase 2 study in newly diagnosed MGMT-unmethylated GBM patients at Sun Yat-sen University Cancer Center in Guangzhou, China. The study is being conducted under its collaboration agreement with Guangxi Wuzhou Pharmaceutical Company.
In this Phase 2 study, VAL-083 is being combined with radiotherapy as a potential replacement for standard-of-care chemoradiation with temozolomide in patients with MGMT-unmethylated GBM. One goal of the study will be to confirm the safety of the three-day VAL-083 dosing regimen in combination with radiotherapy and to investigate outcomes of the combination of VAL-083 and radiotherapy in MGMT-unmethylated GBM patients.
The company plan to enroll up to 30 newly-diagnosed, MGMT-unmethylated GBM patients in this study. The efficacy endpoints of the study include tumor response, as assessed by the Response Assessment in NeuroOncology (“RANO”), and progression-free survival (“PFS”), progression-free survival at six months (“PFS6”), and overall survival (“OS”), compared to historical results in the target population. The study is being conducted in two parts: (1) Dose-confirmation: VAL-083 in cohorts (20, 30 and 40 mg/m2/day IV daily x 3 every 21 days) to assess safety and activity when administered concurrently with x-ray therapy (“XRT”) to confirm the maximum tolerated dose (“MTD”), and (2) Expansion: VAL-083 will be studied in up to 20 additional patients at the target dose, as determined by the dose-confirmation part of the study, administered concurrently with XRT. Assessments of safety and tolerability will be used to support further clinical development of VAL-083 in combination with radiotherapy. Pharmacokinetic assessments of VAL-083 in plasma and cerebral spinal fluid ("CSF") will be used to correlate drug exposure in the central nervous system with patient outcomes.
As of September 21, 2018, nine patients have been enrolled in this study. Dose confirming cohorts studying 20 and 30 mg/m2/day x three every 21 days have been completed and three additional patients have received a VAL-083 starting dose of 40 mg/m2/day x three every 21 days.
The company plan to use data from the study to establish a dosing regimen and study design for advanced registration-directed clinical studies with VAL-083 in newly diagnosed MGMT-unmethylated GBM.
The company anticipate providing updates regarding the progress of this study, including safety data and observations regarding outcomes, at scientific meetings during 2018 and 2019. A detailed description of this study can be found at clinicaltrials.gov, Identifier Number: NCT03050736.
Phase 3: VAL-083 STAR-3 (Avastin-refractory) GBM Study
In July 2017, the company initiated its VAL-083 STAR-3 GBM study as an adaptive, randomized, controlled, pivotal Phase 3 clinical study in patients with GBM whose tumors have progressed following treatment with Avastin (bevacizumab). As previously disclosed in February 2018, based on a number of factors, including low patient enrollment, and its belief that the FDA’s approval of Avastin for rGBM may negatively impact the timely recruitment of suitable patients for this study, the company decided to park this study and undertake a review and assessment of various study parameters. DelMar Pharmaceuticals has now finalized the decision to discontinue this clinical study, other than to support the sole remaining enrolled patient.
Ovarian Cancer
In April 2016, the FDA granted orphan drug designation for the use of VAL-083 in the treatment of ovarian cancer.
In September 2017, the company filed an IND for the use of VAL-083 in ovarian cancer, along with a protocol for a Phase 1/2, open-label, multicenter, study of VAL-083 in patients with Recurrent Platinum Resistant Ovarian Cancer (the REPROVe study).
The FDA has allowed this study to begin enrolling patients, but based on ongoing evaluation and recent input from its newly-formed clinical advisory board, DelMar Pharmaceuticals is reassessing the ovarian cancer program. DelMar Pharmaceuticals is in the process of evaluating the best path forward in ovarian cancer and are looking at various strategic options including combination with PARP inhibitors.
Fast Track Designation
In December 2017, the FDA granted Fast Track designation for VAL-083, in rGBM.
Fast Track designation is designed to expedite the review of drugs that show promise in treating life-threatening diseases and address unmet medical needs, with the goal of getting new treatments to patients earlier. Fast Track designation provides sponsors with an opportunity for increased frequency for communication with the FDA to ensure an optimal development plan and to collect appropriate data needed to support drug approval. Additional benefits of the Fast Track designation may include an Accelerated Approval, a Priority Review, and a Rolling Review. Accelerated Approval is granted to drugs that demonstrate an effect on a surrogate, or intermediate endpoints, reasonably likely to predict clinical benefit. Priority Review shortens the FDA review process for a new drug from ten months to six months and is appropriate for drugs that demonstrate significant improvements in both safety and efficacy of an existing therapy. Rolling Review provides a drug company the opportunity to submit completed sections of its New Drug Application (“NDA”) for review by the FDA. Typically, NDA reviews do not commence until the drug company has submitted the entire application to the FDA. Through the Fast Track designation, the FDA attempts to ensure that questions raised during the drug development process are resolved quickly, often leading to earlier approval and increased access for patients.
VAL-083 Mechanism of Action and the Opportunity in the Treatment of Cancer
Chemotherapy forms the basis of treatment in nearly all cancers. The company believe that VAL-083 may be effective in treating tumors exhibiting biological features that cause resistance to currently available chemotherapy, particularly for patients who have failed, or become resistant to, other treatment regimens.
Based on published research and its own data, the cytotoxic functional groups, and the mechanism of action of VAL-083 are functionally different from alkylating agents commonly used in the treatment of cancer. VAL-083 has previously demonstrated activity in cell-lines that are resistant to other types of chemotherapy. No evidence of cross-resistance has been reported in published clinical studies.
The company's research suggests that VAL-083 attacks cancer cells via a unique mechanism of action which is distinct from other chemotherapies used in the treatment of cancer. The company's data indicate that VAL-083 forms inter-strand crosslinks at the N7 position of guanine on the DNA of cancer cells. The company's data also indicate that this crosslink forms rapidly and is not easily repaired by the cancer cell resulting in cell-cycle arrest and lethal double-strand DNA breaks in cancer cells. VAL-083 readily crosses the blood brain barrier. Published preclinical and clinical research demonstrate that VAL-083 is absorbed more readily in tumor cells than in normal cells.
In vitro, its data also demonstrate that VAL-083’s distinct mechanism may be able to overcome drug resistance against a range of cancers. For example, VAL-083 is active against MGMT-unmethylated GBM cells which are resistant to treatment with temozolomide and nitrosoureas. VAL-083 also retains a high level of activity in p53 mutated non-small cell lung cancer (“NSCLC”), ovarian cancer and medulloblastoma cell lines that are resistant to platinum-based chemotherapy.
Importantly, clinical activity against each of the tumors mentioned above was established in prior NCI-sponsored Phase 2 clinical studies. The company believe that these historical clinical data and its own research support the development of VAL-083 as a potential new treatment for multiple types of cancer.
The main dose-limiting toxicity (“DLT”) related to the administration of VAL-083 in previous NCI-sponsored clinical studies and its own clinical studies is myelosuppression, particularly thrombocytopenia. Myelosuppression, including thrombocytopenia, is a common side effect of chemotherapy. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and causing normal blood clotting. Thrombocytopenia is a reduction in platelet counts which assist in blood clotting. Modern medicine allows for better management of myelosuppressive side effects. The company believe this offers the potential opportunity to improve upon the drug’s already established efficacy profile by substantially increasing the dose of VAL-083 that can be safely administered to cancer patients.
There is no evidence of lung, liver, or kidney toxicity even with prolonged treatment by VAL-083. Data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
Current Treatments for Gliomas and Glioblastoma Multiforme
Gliomas are a type of Central Nervous System (“CNS”) tumor that arises from glial cells in the brain or spine. Glial cells are the cells surrounding nerves. Their primary function is to provide support and protection for neurons in the CNS.
GBM is the most common and the most lethal form of glioma. According to the Central Brain Tumor Registry of The United State, GBM occurs with an incidence of 3.20 per 100,000 person-years. Approximately 13,000 new cases of GBM were diagnosed in the United States and 16,000 in Europe during 2017.
Common symptoms of GBM include headaches, seizures, nausea, weakness, paralysis and personality or cognitive changes such as loss of speech or difficulty in thinking clearly. GBM progresses quickly and patients’ conditions deteriorate rapidly progressing to death. The outlook for GBM patients is generally poor. The overall median survival in newly diagnosed GBM patients with best available treatments is less than 15 months, and two-year and five-year survival rates are approximately 30% and 10%, respectively. Median overall survival in newly-diagnosed, unmethylated GBM patients is 12.2 months.
In September 2017, the National Comprehensive Cancer Network (“NCCN”), updated treatment guidelines for GBM. The recommended treatment regimen for GBM includes surgical resection to remove as much of the tumor as possible (“debulking”) followed by radiotherapy with concomitant and adjuvant chemotherapy with temozolomide with or without tumor treating fields (“TTF”). GBM patients whose tumors exhibit an unmethylated promotor for the gene encoding the DNA repair enzyme MGMT, a biomarker correlated with resistance to temozolomide, may be treated with radiation alone following surgery.
Patients with an unmethylated MGMT promotor have high levels of MGMT, a naturally-occurring DNA repair enzyme that repairs tumor-fighting lesions induced by TMZ thus allowing a patient’s tumor to continue to grow despite treatment which leads to poor outcomes. Measurement of MGMT methylation status has become routine in clinical practice as biomarker that correlates with response to TMZ and patient outcomes in GBM.
Probability of GBM Patient Survival Correlated to Expression of MGMT Enzyme
(Unmethylated promoter = High MGMT Expression and Significantly Shorter Survival)
TTF (Optune®) is a non-invasive technique for adults with GBM. TTF uses alternating electrical fields to disrupt tumor cell division, or cause cell death, thereby preventing the tumor from growing or spreading as quickly. A clinical study reported that GBM patients treated with TTF combined with TMZ experienced longer survival than those treated with TMZ alone.
The majority of GBM patients’ tumors recur within 6 – 12 months of initial treatment. Experimental therapy through clinical studies is recommended under NCCN guidelines for eligible patients. NCCN guidelines also recommend treatment with systemic chemotherapy, such as lomustine (“CCNU”). For patients who are eligible for additional surgical debulking, local chemotherapy with carmustine (“BCNU”) wafers may be employed. CCNU and BCNU target the same DNA-site as TMZ and are also subject to MGMT-related resistance.
Avastin (Avastin®, an anti-VEGF antibody) recently received full approval in the US, Canada, Australia, and Japan as a single agent for patients with recurrent GBM following prior therapy. Avastin carries an FDA “black-box warning” related to severe, sometimes fatal, side effects such as gastrointestinal perforations, wound healing complications and hemorrhage. There are no data demonstrating an improvement in disease-related symptoms or increased survival for GBM patients treated with Avastin.
Recurrent GBM patients, especially those whose tumors progress following treatment with Avastin, have limited or no treatment options and a very poor prognosis. According to published literature, the median survival for GBM patients whose tumors progress following Avastin is less than five months.
VAL-083 Historical Data and The company's Research in GBM
VAL-083 is first-in-class DNA targeting agent that readily crosses the blood-brain-barrier. Data from prior NCI-sponsored clinical studies with VAL-083 demonstrate activity against GBM and other CNS tumors. In general, historical NCI-sponsored studies demonstrate that tumor regression in brain cancer was achieved in 40% of patients treated and stabilization was achieved in an additional 20% to 30% of brain tumor patients following treatment with VAL-083. In these studies, VAL-083 demonstrated statistically significant improvement in the median survival of high-grade glioma brain tumors, including GBM when combined with radiation versus radiation alone (p < 0.05) with results similar, or superior to, other chemotherapies approved for the treatment of GBM.
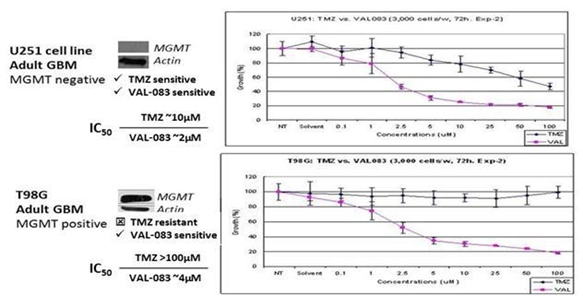
VAL-083 is Active Independent of MGMT
DelMar Pharmaceuticals has presented data at several peer reviewed meetings demonstrating that VAL-083 is active independent of MGMT resistance in GBM cell lines and other CNS tumor cells. The company's research, along with that of others, demonstrates that VAL-083’s unique cytotoxic mechanism forms DNA cross-links at the N7 position of guanine and retains cytotoxic activity independent of MGMT expression in vitro. The company's studies demonstrate that VAL-083 has more potent activity against brain tumor cells in comparison to TMZ and overcomes resistance associated with MGMT, suggesting the potential to surpass the current standard-of-care in the treatment of GBM.
A Summary of The company's Data Demonstrating that VAL-083’s Anti-Tumor Mechanism is Distinct from, and can
Overcome, MGMT-Related Chemo resistance in the Treatment of GBM
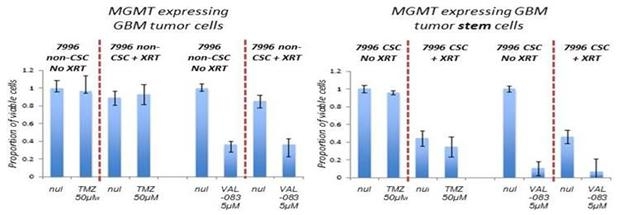
In addition, historical NCI clinical study data and its own research support the activity of VAL-083 as a potentiator of radiotherapy. Radiotherapy in combination with temozolomide is the current standard of care in the treatment of newly diagnosed GBM. The company's research demonstrates that temozolomide and radiotherapy are ineffective against GBM cells exhibiting a high expression of MGMT, whereas VAL-083 potentiates the tumor-killing effect of radiation independent of MGMT expression. Furthermore, the combination of VAL-083 and radiation has been demonstrated to be active against GBM cancer stem cells (“CSCs”) in vitro. CSCs are often resistant to chemotherapy and form the basis for tumor recurrence and metastasis. GBM CSCs display strong resistance to TMZ, even where MGMT expression is low. However, its data demonstrates that GBM CSCs are susceptible to VAL-083 independent of MGMT expression.
A Summary of The company's Data Demonstrating that VAL-083 Maintains Activity in Both Temozolomide-resistant GBM Cell Lines and Matched Cancer Stem Cells and Potentiates Radiotherapy
The company believe that VAL-083’s more potent activity against brain tumor cells in comparison to TMZ, VAL-083’s ability to overcome MGMT-mediated resistance, and its activity against GBM CSCs suggests the potential of VAL-083 to surpass the current standard-of-care in the treatment of GBM.
Phase 1 – 2 Clinical Study Overview and Summary of Results
Forty-eight GBM patients whose disease progressed following prior treatment with temozolomide and Avastin were enrolled in an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics, and anti-cancer activity of VAL-083. The study was conducted at five centers in the United States: The Mayo Clinic in Rochester, Minnesota; the Brain Tumor Center at University of California, San Francisco; the Sarah Cannon Cancer Research Center in Nashville, Tennessee, Denver, Colorado; and the SCRI affiliate site at the Florida Cancer Specialist Research Institute in Sarasota, Florida.
Patients received VAL-083 on days 1, 2 and 3 on a 21-day treatment cycle. The Phase 1 portion of the study involved dose escalation cohorts until a maximum tolerated dose (“MTD”) was established at 40mg/m2. A further 14-patient, Phase 2 expansion was then enrolled at the MTD to gather further safety data at its chosen therapeutic dose and to further explore the outcomes in this patient population.
In May 2016, the company held an end of Phase 2 meeting with the FDA in which the company discussed with the FDA the design of a Phase 3, registration-directed clinical program for VAL-083 in refractory GBM. Based on the input the company received from the FDA, the agency confirmed that it would consider the totality of data available, including data obtained from its other planned clinical studies in related GBM populations, when assessing the NDA. The FDA also noted that the company may be able to rely on prior NCI studies and historical literature to support nonclinical data required for an NDA filing under a 505(b)(2) strategy which allows a sponsor to rely on already established safety and efficacy data in support of an NDA.
The company reported updated results of its Phase 1/2 clinical study at the 2016 ASCO annual meeting. In summary, these data are as follows:
Safety and Tolerability
In the Phase 1 dose escalation regimen, no serious adverse events (“SAE”) related to VAL-083 were encountered at doses up to 40 mg/m2/day.
Increasing frequency of, and higher grade, hematologic toxicities were observed at doses above 40 mg/m2/day. Consistent with the published literature, the observed dose limiting toxicity for VAL-083 is primarily thrombocytopenia (low platelets). Observed platelet nadir occurred at approximately day 18, and recovery was rapid and spontaneous following treatment.
Based on Phase 1 observations, fourteen additional patients were enrolled in a Phase 2 expansion cohort at 40mg/m2, which was established as the MTD. Consistent with Phase 1, the dose of VAL-083 of 40 mg/m2 on days 1, 2 and 3 of a 21-day cycle was generally well tolerated in Phase 2. At this dose, one subject previously treated with CCNU, a nitrosourea agent, reported severe (Grade 4) thrombocytopenia. As a result of this observation, the protocol inclusion criterion for platelet count was increased from 100,000/μL to 150,000/μL for patients receiving prior nitrosoureas within 12 weeks preceding enrollment. No other dose limiting toxicities were observed.
Daily x 5 q 5wks refers to a dosing regimen of once per day for five consecutive days every five weeks (35-day cycle); while daily x 3 q 3wks refers to a dosing regimen of once per day for three consecutive days every three weeks (21-day cycle).
The company's optimized dosing regimen increases the amount of VAL-083 delivered to the CNS by 60% over historical regimens without increased toxicity. Thus, the DelMar regimen achieves both a higher maximum concentration and higher overall exposure, which the company believe may increase the likelihood of successful treatment outcomes in glioblastoma and other brain tumors.
Pharmacokinetics
Pharmacokinetic (“PK”) analyses showed dose-dependent linear systemic exposure with a short (1-2h) plasma terminal half-life; average Cmax at 40 mg/m2/day was 781 ng/mL (5.3µM). The observed PK profile is comparable to published literature. Prior NCI-sponsored studies demonstrated that VAL-083 readily crosses the blood brain barrier and has a long (>20 hour) half-life in the CNS.
The company believe that this PK profile is optimal for the treatment of brain tumors: A long CNS half-life is expected to maximize exposure of the drug in the brain increasing the likelihood of successful treatment outcomes, while a short plasma half-life is desirable to minimize systemic side effects.
Observed pharmacokinetics from VAL-083 Phase 1 clinical study dose vs. AUC
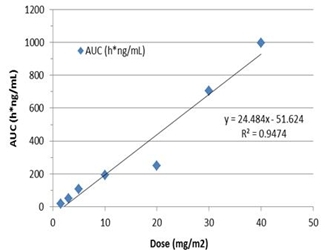
Based on observed and previously published pharmacokinetics, DelMar believes that therapeutic doses equal to, or above, 20 mg/m2 daily on days 1, 2 and 3 of a 21-day cycle should deliver sufficient levels of VAL-083 to brain tumors to achieve a therapeutic benefit.
MGMT & IDH1
High expression of MGMT and wild-type form of the enzyme isocitrate dehydrogenase (“IDH1”) have been previously shown to be diagnostic markers that correlate with resistance to currently available chemotherapies (e.g. temozolomide or nitrosourea) in the treatment of GBM and poor patient outcomes. Measurement of these biomarkers has become routine in clinical practice.
Notably, DelMar Pharmaceuticals has previously demonstrated that VAL-083’s anti-tumor mechanism is active independent from the MGMT status in vitro. The company believe the company will ultimately be able to use such biomarkers in a prognostic fashion to select the patients most likely to respond to treatment as the company expand the clinical development of VAL-083
Tumor Response and Outcomes
rGBM patients in its Phase 1/2 clinical study were not re-resected prior to treatment with VAL-083 and therefore had a growing recurrent GBM tumor at the time of enrollment. Patients were monitored for tumor response by MRI.
Consistent with un-resected rGBM, median progression free survival (“PFS”) was short at 1.2 months (range: 0.2 – 20.1 months). Five rGBM patients treated with VAL-083 were reported to have stable disease as their best response following treatment; the remainder reported progressive disease.
Disease progression is typical in a refractory GBM population with non-resected tumors. However, the company believe that slowed progression may provide meaningful clinical benefit in this patient population through prolonged overall survival and improved quality of life.
According to published literature, GBM patients failing Avastin have a poor prognosis with expected survival under five months. Ad-hoc subgroup analysis of the Phase 1 dose-escalation data indicated a dose response trend and potential for improved survival. Increased survival was observed following initiation of treatment in a high dose (30 and 40mg/m2, n=9) sub-group vs. a low dose (≤5mg/m2, n=6) sub-group with median survival of >9 months vs. 4.4 months for the high and low dose groups, respectively. At the time of the analysis, more than half of patients receiving an assumed therapeutic dose survived more than nine months following Avastin failure; more than 40% survived for ten months and more than 20% survived for twelve months or more.
Observed Survival Based on Phase 1 Sub-Group Analysis
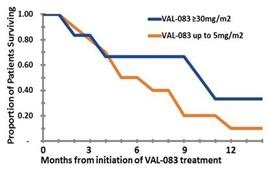
Analysis of twenty-two (22) patients receiving an assumed therapeutic dose of VAL-083 (≥20mg/m2) demonstrated median survival of 8.35 months following Avastin failure.
ASCO 2016: VAL-083 compared to published literature
| Reference | Post Avastin Salvage Therapy | Median Survival following Avastin Failure |
|---|---|---|
| Shih (2016) | VAL-083 | 8.35 months |
| Rahman (2014) | nitrosourea | 4.3 months |
| Mikkelson (2011) | TMZ + irinotecan | 4.5 months |
| Lu (2011) | dasatinib | 2.6 months |
| Reardon (2011) | etoposide | 4.7 months |
| Reardon (2011) | TMZ | 2.9 months |
| Iwomoto (2009) | various | 5.1 months |
While recognizing these data are representative of a relatively small, non-controlled Phase 1/2 clinical study, the company believe these outcomes support the potential of VAL-083 to offer meaningful clinical benefit to GBM patients who have failed Avastin, compared to currently available therapy.
VAL-083 Historical Data and The company's Research in Ovarian Cancer
Ovarian cancer is the fifth most common cancer in women and is the leading cause of death among women diagnosed with gynecological malignancies. In 2016, approximately 22,300 women in the US were diagnosed with ovarian cancer and 14,300 died from their disease.
Without treatment, ovarian cancer spreads within the pelvic region and metastasizes to distant sites such as the lungs, liver, spleen and, rarely, the brain. The initial symptoms of ovarian cancer such as abdominal bloating, indigestion, pelvic pain, or nausea are often attributed to symptoms caused by a less serious condition. Therefore, in most cases, ovarian cancer is not diagnosed until it has progressed to an advanced stage when it is no longer possible to surgically remove all tumor tissue.
When diagnosed at an advanced stage the 5-year survival rate is less than 40%. Women with ovarian cancer receive chemotherapy following surgery to treat residual disease.
VAL-083’s activity against ovarian epithelial adenocarcinoma (“OEA”) and squamous cell carcinoma of the cervix (“SCC”) was reported in prior NCI-sponsored clinical studies. Importantly, NCI-researchers recommended VAL-083 for further advanced studies in the treatment of ovarian cancer.
Pt-based chemotherapy is employed in the treatment of nearly 50% of all cancer patients and is employed in the treatment regimen of nearly all advanced-stage ovarian cancer patients. Ovarian cancer patients whose tumors are sensitive to Pt-based chemotherapy have the most favorable outcome. Recently, the approval of PARP inhibitors in the treatment of ovarian cancer patients demonstrated improved outcomes, particularly patients whose tumors remain sensitive to Pt-based treatments.
Pt-based chemotherapies function by causing extensive damage to a cancer cell’s DNA. Cancer cells are adept at overcoming DNA damage or employing mechanisms to repair DNA damage induced by Pt-based chemotherapy. One of the most common obstacles to DNA-damaging chemotherapy is mutations to a gene called p53. Cellular processes governed by the p53 gene are critical in assessing DNA damage and determining if a cell should cease from dividing or self-destruct. When p53 does not function properly, cancer cells continue to divide despite the treatment with DNA-damaging chemotherapy, making these drugs ineffective and leading to treatment resistance. This occurs in nearly all cases of the most difficult ovarian cancer to treat – high grade serous ovarian cancer (HGSOC) – which accounts for up to 70% of ovarian cancer cases and approximately 90% of ovarian cancer deaths. P53 mutations are associated with resistance to Pt-based chemotherapy, which leads to treatment failure and increased mortality. Solving this problem is a major goal in the development of new treatments for ovarian cancer.
Unfortunately, the development of resistance to Pt-based agents is nearly inevitable, leading to disease recurrence and increased mortality. Ultimately, most women with advanced ovarian cancer develop recurrent disease with progressively shorter disease-free intervals. Those whose tumors recur within 6 months of Pt-based therapy are considered Pt-resistant/refractory and have a very poor prognosis.
The response rate to second line therapy for Pt-resistant ovarian cancer patients is in the 10-15% range and overall survival is approximately 12 months. The development of new chemotherapies and targeted agents to overcome Pt resistance in ovarian cancer is a significant unmet medical need.
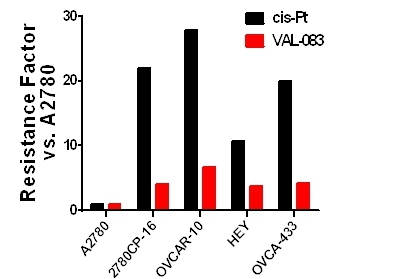
DelMar Pharmaceuticals has presented data demonstrating that VAL-083’s distinct mechanism of action allows activity in tumors that are resistant to other therapies. DelMar Pharmaceuticals has shown that cytotoxicity of VAL-083 against ovarian cancer is independent of sensitivity to cisplatin or p53 status in vitro. DelMar Pharmaceuticals has demonstrated that VAL-083 is active in Pt-resistant ovarian cells harboring a range of p53-mutations.
The company's research has demonstrated that VAL-083 not only overcomes Pt resistance, but the combination of VAL-083 with Pt-based chemotherapy displays synergy in multiple models in vitro and in vivo. This further suggests a distinct mechanism of action and potential use as part of a VAL-083/Pt-combination therapy.
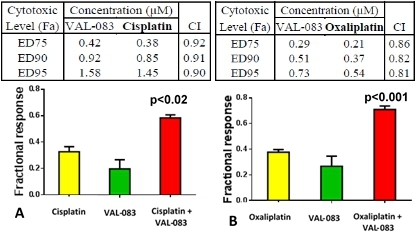
The combination of VAL-083 with either cisplatin (A) or oxaliplatin (B) in the human H460 (WT p53) NSCLC model demonstrated significant super additivity (p≤0.05) and/or synergism (CI<1) for both combinations. This cytotoxic effect of VAL-083 in combination with either platinum drug was observed also in A549 (WT p53) and H1975 (mutant p53) NSCLC cells, independently of p53 status (not shown). Data, where applicable, are shown as mean ± SE; N=7.
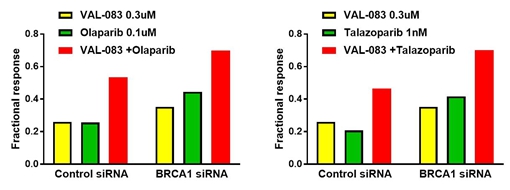
While Pt-based chemotherapy is the standard treatment for ovarian cancer, PARP inhibitors have recently provided a new treatment option for a subset of patients with platinum-sensitive recurrent ovarian cancer. VAL-083 also demonstrates synergistic activity with certain PARP inhibitors, including olaparib (Lynparza) and talazoparib in vitro, suggesting VAL-083 may have utility in the treatment of ovarian cancer in combination with PARP inhibitors.
The company believe that these data demonstrate the potential of VAL-083 to treat platinum-resistant ovarian cancers as a single-agent against platinum-resistant tumors in combination with platinum-based chemotherapeutic regimens or in combination with PARP inhibitors.
Other Indications for VAL-083 – Potential Future Opportunities
VAL-083 in Lung Cancer
Lung cancer is a leading cause of cancer death around the world and effective treatment for lung cancer remains a significant global unmet need despite advances in therapy. Incidence of lung cancer in the United States is approximately 47 per 100,000 with the majority (85%) being NSCLC, the most common type of lung cancer. Globally, the market for lung cancer treatment may exceed $24 billion by 2033 according to a report published by Evaluate Pharma.
The activity of VAL-083 against solid tumors, including lung cancer, has been established in both preclinical and human clinical studies conducted by the NCI. DelMar has developed new nonclinical data to support the utility of VAL-083 in the modern treatment of lung cancer. In an established murine xenograft model of NSCLC, the activity of VAL-083 was compared to standard platinum-based therapy with cisplatin against human NSCLC cell lines A549 (TKI-sensitive) and H1975 (TKI-resistant). In the study, VAL-083 demonstrated superior efficacy and safety in the treatment of TKI-susceptible (A549) tumors and in TKI-resistant (H1975) tumors.
Central Nervous System Metastases of Solid Tumors
The successful management of systemic tumors by modern targeted therapies has led to increased incidence of mortality due to CNS metastases of lung cancer and other solid tumors. In June 2013, the company split its Phase 1/2 clinical study protocol into two separate studies: one focusing solely on refractory GBM and the other focusing on secondary brain cancers caused by other tumors that have spread to the brain.
Based on historical clinical activity and its own research, the company believe that VAL-083 may be suitable for the treatment of patients with CNS metastases who currently have limited treatment options. Subject to the availability of financial and operating resources, the company plan to develop a separate protocol for the continued exploration of VAL-083 in patients with secondary brain cancer caused by a solid tumor spreading to the brain.
Pediatric Brain Tumors
Tumors of the brain and spine make up approximately 20 percent of all childhood cancers and they are the second most common form of childhood cancer after leukemia.
The activity of VAL-083 against childhood and adolescent brain tumors has been established in both preclinical and human clinical studies conducted by the NCI. DelMar Pharmaceuticals has presented data indicating that VAL-083 offers potential therapeutic alternatives for the treatment of pediatric brain tumors including SHH-p53 mutated medulloblastoma. In March 2016, the FDA granted orphan drug designation for the use of VAL-083 in the treatment of medulloblastoma. Subject to the availability of resources, the company intend to collaborate with leading academic researchers for the continued exploration of VAL-083 as a potential treatment of childhood brain tumors.
Additional Indications for VAL-083
In historical studies sponsored by the NCI in the United States, VAL-083 exhibited clinical activity against a range of tumor types including central nervous system tumors, solid tumors, and hematologic malignancies. DelMar Pharmaceuticals has established new nonclinical data supporting the activity of VAL-083 in different types of cancer that are resistant to modern targeted therapies and the company believe that the unique cytotoxic mechanism of VAL-083 may provide benefit to patients in a range of indications. The company intend to continue to research these opportunities, and if appropriate, expand its clinical development efforts to include additional indications.
VAL-083 Target Markets
DNA-targeting agents such as alkylating agents or platinum-based chemotherapy form the mainstay of chemotherapy treatments used in the treatment of cancers. Global sales of platinum-based chemotherapies reached nearly $2.5 billion in 2006 and declined to $600 million following the expiry of key patents. Alkylating agents such as temozolomide, bendamustine, nitrosoureas, and cyclophosphamide generated more than $1.3 billion in sales in 2016 after reaching a peak of $1.7 billion in 2014 (Evaluate Pharma).
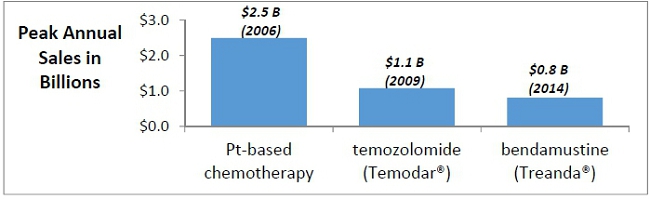
Peak sales of selected DNA-targeting Agents
The company's product candidate, VAL-083, is a first-in-class DNA targeting agent with a novel mechanism of action. VAL-083’s anti-cancer activity was established in a range of tumor types in prior NCI-sponsored clinical studies. Based on this novel mechanism, DelMar Pharmaceuticals has demonstrated that the anti-cancer activity is maintained against tumor cells that are resistant to other DNA-targeting agents. The company believe this positions VAL-083 as a potential chemotherapy-of-choice for patients whose tumors are resistant to current standard-of-care chemotherapy in orphan and major cancer indications.
The company's ongoing research and development activities are focused on indications where VAL-083 demonstrated promising activity in prior NCI-sponsored studies and where its research suggests an opportunity to address significant unmet medical needs due to the failure of existing treatments.
| VAL-083 target markets | 2024 Estimated Global Sales |
|---|---|
| Glioblastoma multiforme (GBM) | 1.5 B |
| Ovarian Cancer | 4.2 B |
| Non-small cell lung cancer (NSCLC) | 32.6 B |
Glioblastoma Multiforme
GBM is the most common and the most lethal form of glioma. According to the Central Brain Tumor Registry of The United State, GBM occurs with an incidence of 3.20 per 100,000 person-years. Approximately 13,000 new cases of GBM were diagnosed in the United States and 16,000 in Europe during 2017.
Newly diagnosed patients suffering from GBM are initially treated through invasive brain surgery, although disease progression following surgical resection is nearly 100%. Temozolomide (Temodar®) in combination with radiation is the front-line therapy for GBM following surgery. Global revenues of branded Temodar reached $1.1 billion in 2009. Following patent expiry in 2013, global revenue for generic temozolomide exceeded $400 million in 2014 even though most patients fail to gain long-term therapeutic benefits. Approximately 60% of GBM patients treated with Temodar® experience tumor progression within one year. Median overall survival in newly-diagnosed, unmethylated GBM patients is 12.2 months.
Bevacizumab (Avastin®) has been approved for the treatment of GBM in patients failing Temodar®. In clinical studies, only about 20% of patients failing Temodar® respond to Avastin® therapy and no improvement in median survival was reported. In spite of these low efficacy results, Avastin revenues exceeded $600 million in 2014.
The market for refractory (Avastin-failed) GBM is limited to those jurisdictions where Avastin is approved for the treatment of GBM. The United States, Canada, Australia, Japan and Switzerland represent the major markets where Avastin is used in the treatment of GBM.
Ovarian Cancer
According to Evaluate Pharma, the annual market for ovarian cancer therapies is projected to exceed $4.2 billion in 2024. The American Cancer Society estimates that approximately 22,000 women will receive a new diagnosis of ovarian cancer and approximately 14,000 women will die from ovarian cancer in the United States each year. Ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system.
The potential of VAL-083 in the treatment of ovarian cancer has been established in prior NCI-sponsored clinical studies and by its recent research. The FDA has granted orphan drug status to VAL-083 as a potential treatment for ovarian cancer and DelMar Pharmaceuticals has recently received notice of allowance for its IND to initiate a Phase 1-2 clinical study to investigate the safety and effectiveness of VAL-083 in patients with recurrent platinum resistant ovarian cancer (VAL-083 REPROVe study).
Ovarian cancers are commonly treated with a platinum-based chemotherapy regimen. Initial tumor response rates are relatively high. However, the development of resistance to Pt-based chemotherapy in ovarian cancer patients is nearly inevitable. The company's research suggests that VAL-083 may offer a potential treatment option for ovarian cancer patients who are resistant to platinum-based chemotherapy and as a potential combination therapy with other agents. The company believe the profile of VAL-083 offers the potential to capture meaningful market share in the multi-billion ovarian cancer market.
Lung Cancer
According to Evaluate Pharma, the annual market for lung cancer therapies is projected to reach nearly $32.6 billion in 2024. Lung cancer is the most common cancer in the world with 1.8 million cases in 2012, representing 13% of all cancers according to a report published by the World Cancer Research Fund International. Lung cancer has a higher mortality rate than the next top three cancers combined and it is responsible for 1.6 million deaths annually, representing 19% of all cancer deaths. NSCLC represents approximately 85% of newly diagnosed lung cancers.
The potential of VAL-083 in the treatment of NSLSC has been established in both human clinical studies conducted by the NCI and by the drug’s commercial approval in China. The company believe the profile of VAL-083 offers the potential to capture meaningful market share in the multi-billion NSCLC market.
VAL-083 Manufacturing
VAL-083 is a small-molecule chemotherapeutic. Chemical synthesis of the active pharmaceutical ingredient (“API”) was initially established by the NCI. DelMar Pharmaceuticals has made improvements to this process and have obtained patents on these improvements. The current manufacturing process involves fewer than five synthetic steps.
VAL-083 drug product is a lyophilized (freeze-dried) formulation that is reconstituted for intravenous injection. The company anticipate that overall cost of goods for an eventual commercial product will be similar to other injectable, small-molecule pharmaceuticals.
DelMar Pharmaceuticals has engaged third-party contract manufacturers with the capabilities to establish the processes, procedures and quality systems necessary to meet U.S., Canadian, E.U. and other international manufacturing requirements in accordance with Good Manufacturing Practice (“cGMP”) regulations.
Supply of VAL-083 for its clinical studies to-date has been provided through a collaboration with Guangxi Wuzhou Pharmaceutical Company. Guangxi Wuzhou Pharmaceutical Company as a manufacturer has established a commercial-scale manufacturing process based on the North American process originally developed for the NCI that has been licensed by the Chinese FDA (“CFDA”) for commercial supply of VAL-083 in China.
DelMar Pharmaceuticals has developed and patented certain intellectual property related to quality controls that are used in the release of VAL-083 for its clinical studies in the United States. This intellectual property is also required for product release under CFDA guidelines and DelMar Pharmaceuticals has granted access to its intellectual property for this purpose.
Research & Development Collaborations
Guangxi Wuzhou Pharmaceutical Company
Pursuant to a memorandum of understanding and collaboration agreement, dated October 25, 2012, DelMar Pharmaceuticals has established a strategic collaboration with Guangxi Wuzhou Pharmaceutical Company, a subsidiary of publicly traded Guangxi Wuzhou Zhongheng Group Co., Ltd. (SHG: 600252) (the “Guangxi Agreement”). VAL-083 is approved for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer in China and Guangxi Wuzhou Pharmaceutical Company is the only manufacturer licensed by the CFDA to produce the product for the China market. Through the Guangxi Agreement, DelMar Pharmaceuticals has been provided drug product at the production price for its VAL-083 clinical studies in the United States and China and DelMar Pharmaceuticals has also secured certain commercial rights in China.
Pursuant to the Guangxi Agreement, the company granted to Guangxi Wuzhou Pharmaceutical Company a royalty-free license to certain of its intellectual property, as it relates to quality control and drug production methods for VAL-083, and the company agreed that Guangxi Wuzhou Pharmaceutical Company will be its exclusive supplier of VAL-083 for clinical studies and commercial sales, subject to Guangxi Wuzhou Pharmaceutical Company obtaining and maintaining cGMP certification by the FDA, EMA or other applicable regulatory agencies, and Guangxi Wuzhou Pharmaceutical Company being able to meet volumes ordered by it. In accordance with this agreement, DelMar Pharmaceuticals has contracted with established third-party suppliers for its Phase 3 clinical studies. The company will continue to work with Guangxi Wuzhou Pharmaceutical Company to achieve FDA compliance in order to potentially have them as its future supplier for global sales of VAL-083.
This Guangxi Agreement also provides it with certain exclusive commercial rights related to drug supply. Specifically, the Guangxi Agreement establishes an exclusive supply relationship between it and Guangxi Wuzhou Pharmaceutical Company for the Chinese market and all markets outside China. Guangxi Wuzhou Pharmaceutical Company agreed that it may not sell VAL-083 for markets outside of China to any other purchaser other than it, provided that, during the first three years following regulatory clearance for marketing of VAL-083 in a particular country or region, the company meet proposed sales volumes set by Guangxi Wuzhou Pharmaceutical Company for the country or region. In addition, Guangxi Wuzhou Pharmaceutical Company granted it a pre-emptive right in China (subject to its acceptance of proposed sales volume and prices) to purchase VAL-083 produced by Guangxi Wuzhou Pharmaceutical Company.
The term of the Guangxi Agreement (except as it relates to the exclusive rights in the China market) is indefinite, subject to termination upon written agreement of all parties, or if either party breaches any material term and fails to remedy such breach within 30 days of receipt of notice of the breach, or if any action to be taken thereunder is not agreed to by both parties, provided that such matter is referred to the chief executive officer of both parties, and they are unable to resolve such matter within 90 days. No payments have been made to date under the Guangxi Agreement.
Duke University Collaboration
In April 2017, the company entered into a three-year collaboration with Duke University to evaluate VAL-083 as a front-line treatment for newly diagnosed patients with GBM. Under the terms of the collaboration, the company will fund a series of preclinical studies to be conducted by Duke University’s Glioblastoma Drug Discovery Group to identify molecular characteristics of GBM tumors that are more likely to respond to VAL-083, and not the standard of care, temozolomide, as a front-line treatment or through combination therapies.
Patents and Proprietary Rights
The company's success will depend in part on its ability to protect its existing product candidate and the products the company acquire or license by obtaining and maintaining a strong proprietary position. To develop and maintain its position, the company intend to continue relying upon patent protection, orphan drug status, Hatch-Waxman exclusivity, trade secrets, know-how, continuing technological innovations and licensing opportunities.
DelMar Pharmaceuticals has filed patent applications claiming the use of, and improvements related to VAL-083. The company's patent filings also include proposed treatment regimens, improvements to the manufacturing process, formulation and composition of the active pharmaceutical ingredient, and finished dosage forms of VAL-083. DelMar Pharmaceuticals is prosecuting its patent applications in the United States and other jurisdictions which the company deem important for the potential commercial success of VAL-083.
One of the inventors listed in its Series IX applications is an employee of the University of California, San Francisco. If a patent issues from a patent application in this series with a claim that the University of California employee conceived of, in whole or in part, then the Regents of the University of California will share ownership of any such patent with it. The company's research agreements with the University of California address this issue by providing it with an exclusive option, for a limited period of time, to negotiate a royalty-bearing exclusive license for commercialization of the invention covered by that patent.
In addition to patent protection, the company may also seek orphan drug status whenever it is available. If a product which has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the U.S. and Canada, and 10 years in the E.U. Orphan drug designation does not prevent competitors from developing or marketing different drugs for the same indication or the same drug for a different clinical indication.
In February 2012, the FDA granted orphan drug status to VAL-083 for the treatment of glioma. In January 2013, the EMA also granted orphan drug protection to VAL-083 for the treatment of glioma. In the spring of 2016, the FDA Office of Orphan Products Development granted orphan drug designations to VAL-083 for the treatment of ovarian cancer and medulloblastoma.
In addition to its patents and orphan drug protection, the company intend to rely on the Hatch-Waxman Amendments for five years of data exclusivity for VAL-083.Under the Hatch-Waxman Amendments, newly approved drugs and indications benefit from a statutory period of non-patent marketing exclusivity. These amendments provide five-year data exclusivity to the first applicant to gain approval of an NDA for a new chemical entity, meaning that the FDA has not previously approved any other new drug containing the same active ingredient. The Hatch-Waxman Amendments prohibit the approval of an abbreviated new drug application, also known as an ANDA or generic drug application, during the five-year exclusive period if no patent is listed. If there is a patent listed and the ANDA applicant certifies that the NDA holder’s listed patent for the product is invalid or will not be infringed, the ANDA can be submitted four years after NDA approval. Protection under the Hatch-Waxman Amendments will not prevent the filing or approval of another full NDA; however, the applicant would be required to conduct its own pre-clinical studies and adequate and well-controlled clinical studies to demonstrate safety and effectiveness. The Hatch-Waxman Amendments also provide three years of data exclusivity for the approval of NDAs with new clinical studies for previously approved drugs and supplemental NDAs, for example, for new indications, dosages or strengths of an existing drug, if new clinical investigations were conducted by or on behalf of the sponsor and were essential to the approval of the application. This three-year exclusivity covers only the new changes associated with the supplemental NDA and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient.
The company also rely on trade secret protection for its confidential and proprietary information. The company believe that the substantial costs and resources required to develop technological innovations, such as the manufacturing processes associated with VAL-083, will help it to protect the competitive advantage of its product candidate.
The protection of intellectual property rights in China (where its clinical product candidate, VAL-083, is manufactured pursuant to a collaboration agreement with the only manufacturer presently licensed by the CFDA to produce the product for the China market, and where VAL-03 is approved for the treatment of CML and lung cancer) is relatively weak compared to the United States, which may negatively affect its ability to generate revenue from VAL-083 in China.
The company's policy is to require its employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with it. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with it is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees and consultants, the agreements provide that all inventions conceived by the individual shall be its exclusive property.




