Johnson & Johnson
Summary
- Johnson & Johnson primary focus is products related to human health and well-being.
- Johnson & Johnson is a holding company, with operating companies conducting business in virtually all countries of the world.
- Recently Johnson & Johnson completed acquisition of Abiomed

Johnson & Johnson (NYSE: JNJ) has approximately 152,700 employees worldwide engaged in the research and development, manufacture and sale of a broad range of products in the healthcare field. Johnson & Johnson is a holding company, with operating companies conducting business in virtually all countries of the world. The Company’s primary focus is products related to human health and well-being. Johnson & Johnson was incorporated in the State of New Jersey in 1887.
Recent Developments
Janssen Reports Positive Topline Phase 2 Results for Nipocalimab1
February 6, 2023; The Janssen Pharmaceutical Companies of Johnson & Johnson today announced positive topline results from the proof-of-concept Phase 2 open-label UNITY clinical trial for the treatment of pregnant adults at high risk for severe hemolytic disease of the fetus and newborn (HDFN). HDFN is a serious and rare condition which can cause life-threatening anemia in the fetus. It occurs when the blood types of a pregnant individual and their fetus are incompatible.1 The trial met the primary endpoint, with the majority of pregnant patients who received nipocalimab achieving a live birth at or after the gestational age (GA) of 32 weeks, without the need for an intrauterine transfusion (IUT) throughout their entire pregnancy. During the treatment period of approximately 20 weeks, nipocalimab demonstrated a safety profile that supports further development of the treatment in HDFN.
Nipocalimab was granted Fast Track designation in July 2019 and orphan drug status in June 2020 by the U.S. Food and Drug Administration (FDA), and orphan medicinal product designation by the European Medicines Agency in October 2019 for HDFN.
Johnson & Johnson Completes Acquisition of Abiomed2
December 22, 2022; Johnson & Johnson (NYSE: JNJ), the world’s largest, most diversified healthcare products company, today announced it has completed its acquisition of Abiomed, Inc. Abiomed is now part of Johnson & Johnson and will operate as a standalone business within Johnson & Johnson’s MedTech segment.
Johnson & Johnson’s tender offer for all outstanding shares of Abiomed for an upfront payment of $380.00 per share in cash, corresponding to an enterprise value of approximately $16.6 billion. This acquisition allows Johnson & Johnson MedTech to expand its portfolio in the high growth cardiovascular markets, adding solutions for heart recovery to its global market leading Biosense Webster electrophysiology business. Fueled by Johnson & Johnson’s global scale and commercial and clinical strength, JNJ is excited to explore the opportunities and possibilities ahead to reach even more patients with critical unmet need.

Financial Highlights
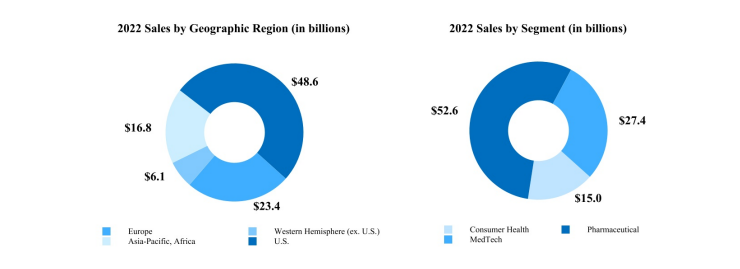
In 2022, worldwide sales increased 1.3% to $94.9 billion as compared to an increase of 13.6% in 2021. Sales by U.S. companies were $48.6 billion in 2022 and $47.2 billion in 2021. This represents increases of 3.0% in 2022 and 9.3% in 2021. Sales by international companies were $46.4 billion in 2022 and $46.6 billion in 2021. This represents a decrease of 0.6% in 2022 and an increase of 18.2% in 2021.3
In 2022, sales by companies in Europe experienced a decline of 0.6% as compared to the prior year, which included operational growth of 11.0% and a negative currency impact of 11.6%. Sales by companies in the Western Hemisphere, excluding the U.S., achieved growth of 6.5% as compared to the prior year, which included operational growth of 10.2%, and a negative currency impact of 3.7%. Sales by companies in the Asia-Pacific, Africa region experienced a decline of 2.8% as compared to the prior year, including operational growth of 6.2% and a negative currency impact of 9.0%.
Consumer Health segment sales in 2022 were $15.0 billion, a decrease of 0.5% from 2021, which included 3.6% operational growth and a negative currency impact of 4.1%. U.S. Consumer Health segment sales were $6.6 billion, an increase of 1.3%. International sales were $8.4 billion, a decrease of 1.9%, which included 5.3% operational growth and a negative currency impact of 7.2%. In 2022, acquisitions and divestitures had a net negative impact of 0.3% on the operational sales growth of the worldwide Consumer Health segment.
Pharmaceutical segment sales in 2022 were $52.6 billion, an increase of 1.7% from 2021, which included operational growth of 6.7% and a negative currency impact of 5.0%. U.S. sales were $28.6 billion, an increase of 2.3%. International sales were $24.0 billion, an increase of 1.0%, which included 11.9% operational growth and a negative currency impact of 10.9%. In 2022, acquisitions and divestitures had a net negative impact of 0.1% on the operational sales growth of the worldwide Pharmaceutical segment. Adjustments to previous sales reserve estimates were approximately $0.1 billion and $0.7 billion in fiscal years 2022 and 2021, respectively.
The MedTech segment sales in 2022 were $27.4 billion, an increase of 1.4% from 2021, which included operational growth of 6.2% and a negative currency impact of 4.8%. U.S. sales were $13.4 billion, an increase of 5.4% as compared to the prior year. International sales were $14.1 billion, a decrease of 2.3% as compared to the prior year, which included operational growth of 6.9% and a negative currency impact of 9.2%. In 2022, the net impact of acquisitions and divestitures on the MedTech segment worldwide operational sales growth was a positive 0.1%.
Cash and cash equivalents were $14.1 billion at the end of 2022 as compared to $14.5 billion at the end of 2021.
Company Overview
Johnson & Johnson and its subsidiaries have approximately 152,700 employees worldwide engaged in the research and development, manufacture and sale of a broad range of products in the healthcare field. Johnson & Johnson is a holding company, with operating companies conducting business in virtually all countries of the world. The Company’s primary focus is products related to human health and well-being. Johnson & Johnson was incorporated in the State of New Jersey in 1887.
Business Segments
The Company is organized into three business segments: Consumer Health, Pharmaceutical and MedTech.
Consumer Health
The Consumer Health segment includes a broad range of products focused on personal healthcare used in the Skin Health/Beauty, Over-the-Counter medicines, Baby Care, Oral Care, Women’s Health and Wound Care markets. Major brands in Skin Health/Beauty include the AVEENO; CLEAN & CLEAR; DR. CI:LABO; NEUTROGENA and OGX product lines. Over-the-Counter (OTC) medicines include the broad family of TYLENOL acetaminophen products; SUDAFED cold, flu and allergy products; BENADRYL and ZYRTEC allergy products; MOTRIN IB ibuprofen products; NICORETTE smoking cessation products outside the U.S.; ZARBEE’S products, inspired by nature, and the PEPCID line of acid reflux products. Baby Care includes the JOHNSON’S and AVEENO Baby line of products. Oral Care includes the LISTERINE product line. Major brands in Women’s Health outside of North America are STAYFREE and CAREFREE sanitary pads and o.b. tampon brands. Wound Care brands include the BAND-AID Brand Adhesive Bandages and NEOSPORIN First Aid product lines. These products are marketed to the general public and sold online (eCommerce) and to retail outlets and distributors throughout the world.
In November 2021, the Company announced its intention to separate the Company’s Consumer Health business (Kenvue as the name for the planned New Consumer Health Company), with the intention to create a new, publicly traded company by the end of the fiscal year 2023.
Pharmaceutical
The Pharmaceutical segment is focused on the following therapeutic areas: Immunology (e.g., rheumatoid arthritis, psoriatic arthritis, inflammatory bowel disease and psoriasis), Infectious Diseases (e.g., HIV/AIDS), Neuroscience (e.g., mood disorders, neurodegenerative disorders and schizophrenia), Oncology (e.g., prostate cancer, hematologic malignancies, lung cancer and bladder cancer), Cardiovascular and Metabolism (e.g., thrombosis, diabetes and macular degeneration) and Pulmonary Hypertension (e.g., Pulmonary Arterial Hypertension). Medicines in this segment are distributed directly to retailers, wholesalers, distributors, hospitals and healthcare professionals for prescription use. Key products in the Pharmaceutical segment include: REMICADE (infliximab), a treatment for a number of immune-mediated inflammatory diseases; SIMPONI (golimumab), a subcutaneous treatment for adults with moderate to severe rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis and moderately active to severely active ulcerative colitis; SIMPONI ARIA (golimumab), an intravenous treatment for adults with moderate to severe rheumatoid arthritis, active psoriatic arthritis and active ankylosing spondylitis and active polyarticular juvenile idiopathic arthritis (pJIA) in people 2 years of age and older; STELARA (ustekinumab), a treatment for adults and children with moderate to severe plaque psoriasis, for adults with active psoriatic arthritis, for adults with moderately to severely active Crohn's disease and treatment of moderately to severely active ulcerative colitis; TREMFYA (guselkumab), a treatment for adults with moderate to severe plaque psoriasis and active psoriatic arthritis; EDURANT (rilpivirine), PREZISTA (darunavir) and PREZCOBIX/REZOLSTA (darunavir/cobicistat), antiretroviral medicines for the treatment of human immunodeficiency virus (HIV-1) in combination with other antiretroviral products and SYMTUZA (darunavir/cobicistat/emtricitabine/tenofovir alafenamide), a once-daily single tablet regimen for the treatment of HIV; CONCERTA (methylphenidate HCl) extended-release tablets CII, a treatment for attention deficit hyperactivity disorder; INVEGA SUSTENNA/XEPLION (paliperidone palmitate), for the treatment of schizophrenia and schizoaffective disorder in adults; INVEGA TRINZA/TREVICTA (paliperidone palmitate), for the treatment of schizophrenia in patients after they have been adequately treated with INVEGA SUSTENNA for at least four months; RISPERDAL CONSTA (risperidone long-acting injection), for the treatment of schizophrenia and the maintenance treatment of Bipolar 1 Disorder in adults; ZYTIGA (abiraterone acetate), a treatment for patients with prostate cancer; ERLEADA (apalutamide), a next-generation androgen receptor inhibitor for the treatment of patients with prostate cancer; IMBRUVICA (ibrutinib), a treatment for certain B-cell malignancies, or blood cancers and chronic graft versus host disease; DARZALEX (daratumumab), a treatment for multiple myeloma; DARZALEX FASPRO (daratumumab and hyaluronidase-fihj), a treatment for multiple myeloma and light chain (AL) Amyloidosis; XARELTO (rivaroxaban), an oral anticoagulant for the prevention of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE) in patients undergoing hip or knee replacement surgery, to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, and for the treatment and reduction of risk of recurrence of DVT and PE to reduce the risk of major cardiovascular events in patients with coronary artery disease (CAD) and peripheral artery disease (PAD), for the treatment and secondary prevention of thromboembolism in pediatric patients, and for thromboprophylaxis in pediatric patients following the Fontan procedure; INVOKANA (canagliflozin), for the treatment of adults with type 2 diabetes; INVOKAMET/VOKANAMET (canagliflozin/metformin HCl), a combination therapy of fixed doses of canagliflozin and metformin hydrochloride for the treatment of adults with type 2 diabetes; and INVOKAMET XR (canagliflozin/metformin hydrochloride extended-release), a once-daily, fixed-dose combination therapy of canagliflozin and metformin hydrochloride extended-release, for the treatment of adults with type 2 diabetes; OPSUMIT (macitentan) as monotherapy or in combination, indicated for the long-term treatment of pulmonary arterial hypertension (PAH); UPTRAVI (selexipag), the only approved oral and intravenous, selective IP receptor agonist targeting a prostacyclin pathway in PAH. Many of these medicines were developed in collaboration with strategic partners or are licensed from other companies and maintain active lifecycle development programs.
MedTech
The MedTech (previously referred to as Medical Devices) segment includes a broad portfolio of products used in the Interventional Solutions, Orthopaedics, Surgery and Vision categories. Interventional Solutions include Electrophysiology products (Biosense Webster) to treat cardiovascular diseases, Neurovascular care (Cerenovus) that treats hemorrhagic and ischemic stroke and the Heart Recovery portfolio (Abiomed) which includes technologies to treat severe coronary artery disease requiring high-risk PCI or AMI cardiogenic shock. The Orthopaedics portfolio (DePuy Synthes) comprises products in support of Hips, Knees, Trauma, and Spine, Sports & Other. The Surgery portfolios include advanced and general surgery offerings (Ethicon), solutions that focus on Breast Aesthetics (Mentor), and Ear, Nose and Throat (Acclarent) procedures. Johnson & Johnson Vision products include ACUVUE Brand contact lenses and ophthalmic technologies related to cataract and laser refractive surgery. These products are distributed to wholesalers, hospitals and retailers, and used predominantly in the professional fields by physicians, nurses, hospitals, eye care professionals and clinics.

Brands and Products
| Sefl Care | Skin Health/Beauty | Essential Health | Medical Devices | Pharmaceuticals |
| Tylenol | NEUTROGENA | Listerine | Acclarent | Actelion |
| Motrin | AVEENO | BAND-AID | Animas Corporation | Janssen |
| Zyrtec | Dr. Gi Labo | Johnson's | Biosense Webster | Janssen-Cilag |
| Benadryl | NeoStrata | Aveeno Baby | Codman & Shurtleff | Ortho Biotech |
| Zarbee's | Dabao | Carefree | DePuy Synthes | Tibotec |
| Nicorette | OGX | Desitin | Ethicon | Janssen Vaccines |
| Calpol | Le Petit Marseillais | Neosporin | Mentor Worldwide | |
| Codral | Bebe Young Care | O.B. | NeuWave Medical | |
| Doktor Mom | Clean & Clear | Penaten | SterilMed | |
| Dolormin | Exuviance | Polysporin | Vistakon Pharmaceuticals | |
| Frenadol | Labo Labo | Stayfree | ||
| Imodium | Lubriderm | |||
| ORSL | Piz Buin | |||
| Pepcid | Regaine | |||
| Sudafed | Rogaine | |||
| Visine | Sundown |
Manufacturing Facilities
The locations of the manufacturing facilities by major geographic areas of the world
Within the U.S.,
- 4 facilities are used by the Consumer Health segment,
- 5 by the pharmaceutical segment and
- 19 by the MedTech segment.
Outside of the U.S.,
- 23 facilities are used by the Consumer Health segment,
- 13 by the pharmaceutical segment and
- 25 by the MedTech segment.
Company History
Johnson & Johnson's some of the significant events and milestones4
| Year | Milestone |
| 1886 | Johnson & Johnson is founded in New Brunswick, New Jersey by brothers Robert, James, and Edward Johnson. |
| 1888 | The company introduces its first commercial product, antiseptic surgical dressings. |
| 1894 | Johnson & Johnson begins manufacturing first aid kits for use by railroad workers. |
| 1907 | The company establishes its first international subsidiary in Canada. |
| 1921 | Johnson & Johnson introduces the first commercial first aid cream, which becomes the basis for the company's Neosporin product line. |
| 1931 | The company enters the dental market with the acquisition of dental products manufacturer, Chiclets. |
| 1944 | Johnson & Johnson creates the world's first first aid manual for use by the general public. |
| 1959 | The company launches its first consumer healthcare product, baby shampoo. |
| 1961 | Johnson & Johnson introduces Tylenol, a pain reliever that becomes one of the company's most successful products. |
| 1982 | Johnson & Johnson becomes the first healthcare company to make the Fortune 500 list of America's largest corporations. |
| 1987 | The company introduces the world's first disposable contact lenses. |
| 1995 | Johnson & Johnson acquires Cordis Corporation, a manufacturer of medical devices. |
| 2002 | The company is listed on the Dow Jones Sustainability Index. |
| 2011 | Johnson & Johnson is recognized as the world's most respected company in the pharmaceutical industry by Barron's magazine. |
| 2020 | The company begins development of a COVID-19 vaccine, which is granted emergency use authorization by the FDA in February 2021.
|
References
- ^ https://www.jnj.com/janssen-reports-positive-topline-phase-2-results-for-nipocalimab-in-pregnant-individuals-at-high-risk-for-severe-hemolytic-disease-of-the-fetus-and-newborn-hdfn
- ^ https://johnsonandjohnson.gcs-web.com/static-files/833ebed0-a806-4be2-ba8c-0b013fe5de84
- ^ https://fintel.io/doc/sec-johnson-johnson-200406-10k-2023-february-16-19404-2413
- ^ https://ourstory.jnj.com/our-beginning#experience-our-beginning




