Marinus Pharmaceuticals
Overview
Marinus Pharmaceuticals (MRNS) is a clinical stage biopharmaceutical company focused on developing and commercializing innovative therapeutics to treat epilepsy and neuropsychiatric disorders. The company's clinical stage product candidate, ganaxolone, is a positive allosteric modulator of GABAA being developed in three different dose forms: intravenous (IV), capsule and liquid. The multiple dose forms are intended to maximize the therapeutic range of ganaxolone for adult and pediatric patient populations, in acute and chronic care, and both in-patient and self-administered settings. Ganaxolone exhibits anti-seizure, anti-anxiety and anti-depressive actions via its effects on synaptic and extrasynaptic GABAA receptors.1
Pipeline
Marinus Pharmaceuticals is developing ganaxolone to treat children suffering from rare epilepsies and both adults and children suffering from other neuropsychiatric conditions where there is a mechanistic rationale for ganaxolone to provide a benefit, including the following indications:
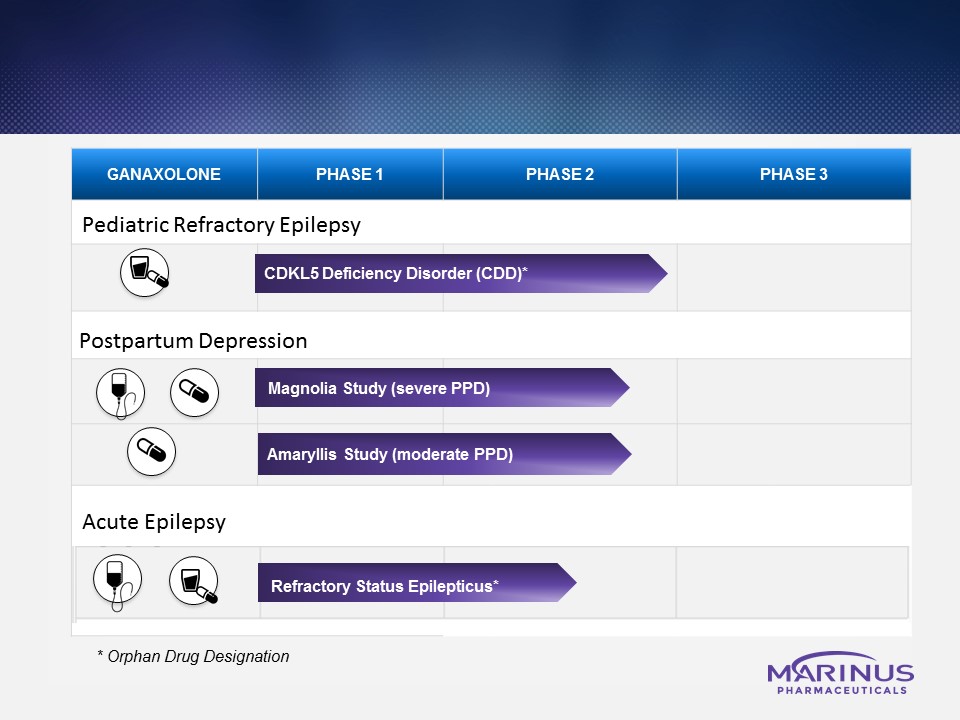
CDKL5 deficiency disorder (CDD)
CDD is a serious and rare genetic disorder that is caused by a mutation of the cyclin-dependent kinase-like 5 (CDKL5) gene, located on the X chromosome. It predominantly affects girls and is characterized by early-onset, difficult-to-control seizures and severe neuro‑developmental impairment. The CDKL5 gene encodes proteins essential for normal brain function. Most children affected by CDD cannot walk normally, talk, or care for themselves. Many also suffer from scoliosis, visual impairment, gastrointestinal difficulties, and sleeping disorders. Currently, there are no approved therapies for CDD. The company believe that no previous late-stage clinical trials have been conducted in this patient population.
In September 2017, the company announced Phase 2 results in patients suffering from CDD. Patients in the CDD cohort of the Phase 2 open-label study in orphan pediatric epilepsies showed a median decrease of 43% (n=7) in 28-day seizure frequency from baseline in the ITT (intent-to-treat) population (primary endpoint). The median change from baseline in seizure-free days in the ITT population (key secondary endpoint) was an increase of 78% (n=5; two subjects cannot be calculated due to 0 baseline seizure-free days). The majority of patients continue to receive ganaxolone and have entered the one-year extension of the study. Ganaxolone was generally safe and well-tolerated with no serious adverse events.
To date, there have been no adverse event reports of somnolence or dizziness and two children discontinued prior to completing the 26-week treatment due to lack of efficacy. Marinus Pharmaceuticals has met with The United States Food and Drug Administration (FDA) and plan to meet with foreign regulators with the goal of commencing the Phase 3 clinical study in mid-2018.
The FDA granted Orphan Drug Designation to ganaxolone for the treatment of CDD. Orphan Drug Designation is granted by the FDA Office of Orphan Products Development (OOPD) to novel drugs or biologics that treat a rare disease or condition affecting fewer than 200,000 patients in the U.S. The designation provides the drug developer with a seven-year period of U.S. marketing exclusivity, as well as tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance and waiver of Prescription Drug User Fee Act (PDUFA) filing fees.
Postpartum Depression (PPD)
PPD is a mood disorder that affects about 15% of women within the first year following childbirth. Common symptoms include feelings of extreme sadness, hopelessness, suicidal ideation, anxiety, and fatigue. PPD is thought to be linked to the rapid fluctuations in the levels of reproductive hormones and allopregnanolone (allo) after childbirth. Allo has been shown in clinical studies to be effective in treating patients with severe PPD. PPD can affect a mother’s ability to care for her child and may negatively affect a child’s cognitive development. There are no approved treatments for PPD, but the most common treatments are psychotherapy and antidepressants. The company believe that treatment with ganaxolone may provide benefit to women suffering from PPD.
In June 2017, the company initiated a Phase 2 double-blind, placebo-controlled clinical trial to evaluate the safety, efficacy and pharmacokinetics (PK) of ganaxolone IV in women diagnosed with severe PPD (Magnolia study). Patients randomized in the initial cohort(s) will undergo an infusion of either ganaxolone or placebo and will be followed for 30 days. Subsequent Magnolia study cohorts could include intravenous regimens of various durations alone or in sequential administration with oral ganaxolone. In December 2017, the company initiated a Phase 2 study to evaluate the safety, tolerability and efficacy of ganaxolone oral capsules in moderate PPD patients (Amaryllis study). Preliminary data from Magnolia and Amaryllis studies are expected in 2018 and will be used to inform later stage development of ganaxolone in PPD.
Status Epilepticus (SE)
SE is a life-threatening occurrence of continuous or intermittent seizures lasting more than five minutes in duration without full recovery. If SE is not treated immediately, permanent neuronal damage may occur, which contributes to high rates of morbidity and mortality. In refractory status epilepticus (RSE), certain synaptic GABAA receptors are internalized, and thereby unavailable to drugs that target these receptors, such as benzodiazepines. According to LexisNexis, there are approximately 45,000 cases of hospitalized RSE treated in the United States annually. RSE patients who do not respond to additional antiepileptic drugs (AEDs), referred to as having super refractory status epilepticus (SRSE), are generally placed under IV anesthesia as a last resort to attempt to stop the seizures and prevent further damage to the brain and death.
Ganaxolone modulates both synaptic and extrasynaptic GABAA receptors, allowing a therapeutic pathway in situations where synaptic GABAA receptors are unavailable. Ganaxolone has shown activity at least comparable to allo in preclinical rat models of benzodiazepine-resistant SE. Another preclinical rat model of benzodiazepine refractory SE showed anti-epileptic synergy with the combination of ganaxolone and diazepam in blocking pilocarpine-induced seizures in rats. Ganaxolone and diazepam plasma levels were identical when measured both alone and in combination, indicating that neither drug affected the pharmacokinetic disposition of the other. These data may have clinical implications on the treatment and dosing of ganaxolone in patients with SE who are or have been treated with benzodiazepines.
Marinus Pharmaceuticals has initiated a Phase 2 feasibility study with ganaxolone IV in patients with RSE. The Phase 2 trial is designed to treat patients in the SE treatment paradigm as second line when they have active brain function and potential for better outcomes. Preliminary data from this feasibility study are expected in 2018.
In April 2016, the FDA granted Orphan Drug Designation to the IV formulation of ganaxolone for the treatment of SE.
Other Indications
Marinus Pharmaceuticals has also conducted proof-of-concept studies in Lennox Gastaut Syndrome (LGS), PCDH19 pediatric epilepsy (PCDH19-PE) and Fragile X Syndrome (FXS). Data from these studies showed that ganaxolone was safe and well-tolerated and effective in either reducing seizures or improving anxiety. Children with LGS, PCDH19-PE and FXS often suffer from cognitive and developmental impairment, behavioral challenges, sleep disorders and seizures. Ganaxolone could be helpful to these patients across a range of these co-morbidities. The company may study these and/or other indications in future clinical trials.
Ganaxolone Mechanism of Action
Ganaxolone is a synthetic analog of a naturally occurring neurosteroid, allo, which exhibits potent anxiolytic, antidepressant, antiepileptic and sedative activity by virtue of its GABAA receptor modulating properties. While allo’s activities are well documented, allo has the potential to convert back to its metabolic precursor progesterone, which could lead to hormonal side effects. Ganaxolone has been designed with an added methyl group that prevents back conversion to an active steroid, which the company believe unlocks ganaxolone’s potential for chronic use. This engineering also enables ganaxolone to be dosed orally. In preclinical studies, ganaxolone has exhibited potency and efficacy comparable to allo.
GABA (gamma-aminobutyric acid) is the chief inhibitory neurotransmitter in the brain. One of the subclasses of receptors that respond to GABA is the GABAA receptor. When activated, these receptors selectively conduct chloride ions through a pore that results in the inhibitory effect of hyperpolarization of the neuron. Synaptic GABAA receptors respond quickly to inhibit neurotransmission, while extrasynaptic GABAA receptors provide ambient tonic inhibition.
Ganaxolone and allo interact with both synaptic and extrasynaptic GABAA receptors and at binding sites distinct from the benzodiazepines. Activity with extrasynaptic GABAA receptors may be of particular importance for treating patients who developed tolerance to benzodiazepines and barbiturates. Ganaxolone binds to the GABAA receptors, which opens the pore to allow chloride ions to move into the postsynaptic neuron, leading to the inhibition of neurotransmission.
Safety Overview
Oral Safety
More than 1,600 subjects have received oral treatment with ganaxolone ranging in duration from one day to more than two years using doses from 50 to 2,000 mg/day. Ganaxolone was administered in Phase 2 studies to pediatric subjects at doses up to 63 mg/kg and to adult subjects at doses up to 1,875 mg/day. No drug-related deaths occurred in any of these clinical trials, and the majority of adverse events were not medically serious and resolved upon discontinuation of therapy. The most common side effects are related to sedation. In the ganaxolone safety database there are no trends of medically important changes in blood chemistry, vital signs, liver function, renal function or cardiovascular parameters in the adult or pediatric populations.
IV Safety
In 2016, the company completed a Phase 1 dose-escalation study in ganaxolone IV. In this study, the company achieved dose levels targeted for efficacy in patients with postpartum depression (PPD), status epilepticus (SE) and other indications. The Phase 1 clinical study enrolled 36 subjects and was designed to determine the pharmacokinetics (PK), pharmacodynamics (PD), and safety of ganaxolone IV administered as an ascending bolus dose (Stage 1) or continuous infusion (Stage 2). Four cohorts of subjects were enrolled in Stage 1 and received escalating doses of ganaxolone, and one cohort of subjects was enrolled in Stage 2.
In the study, every dose regimen of ganaxolone IV administered, either bolus or continuous infusion, was generally safe and well tolerated, and reached targeted dose levels in a short period of time. Following treatment, six treatment-emergent adverse events were reported, all of which were mild in severity and resolved without intervention. Only headache was considered possibly related to treatment with ganaxolone IV. No subject discontinued due to an adverse event and no serious adverse events were reported. Ganaxolone IV plasma concentrations were generally proportional to the administered dose. Additionally, the continuous infusion of ganaxolone IV achieved the targeted exposure levels. Plasma exposures associated with anticonvulsant and anti-anxiety activity were reached in this study.
Preclinical Pharmacology and Toxicology
Marinus Pharmaceuticals has completed preclinical safety pharmacology and toxicology testing, including reproductive toxicology. Animal pharmacokinetic and in vitro studies show that ganaxolone is primarily metabolized by the CYP3A family of liver enzymes, a common route of drug metabolism. All in vitro studies have shown ganaxolone has low potential for interaction with other drugs at several multiples of observed human ganaxolone levels. Furthermore, neither ganaxolone nor its metabolites have a ketone ring at the 3-position, a requirement for hormonal activity. In binding studies, ganaxolone has no appreciable affinity for estrogen or progesterone receptors. The company found no evidence of changes in blood, liver, kidney or the gastrointestinal systems indicating functional or anatomical adverse effects associated with either single- or multiple-dose treatment with ganaxolone in preclinical safety pharmacology studies, nor have the company seen evidence of any end organ toxicity from human clinical studies. Marinus Pharmaceuticals has not detected potential for ganaxolone to cause cellular mutations or carcinogenicity in studies to date.
In reproductive toxicology studies, ganaxolone did not cause any malformations of the embryo or fetus in rats or mice and did not significantly affect the development of offspring. No changes in sperm parameters were found. The company believe these findings are important as all currently marketed AEDs have shown developmental toxicities in animal studies such as fetal death or skeletal abnormalities that indicates a finding of developmental toxicities in animal studies. Valproate, carbamazepine, phenytoin and topiramate have been linked with birth defects in humans (e.g. head and facial malformations and lowered birth weight) at a rate higher than observed in women who did not take these drugs. This association has resulted in labeling for these drugs indicating positive evidence of human fetal risk based on scientific data. Based on ganaxolone’s mechanism and preclinical and clinical findings to date, the company intend to seek differentiated labeling for ganaxolone, indicating that animal reproduction studies have failed to demonstrate a risk to the fetus, which the company believe would be an important safety differentiator for women of childbearing age.
Strategy
The company's goal is to maximize the value of ganaxolone as a best‑in‑class innovative neuropsychiatric therapy with a portfolio of diversified indications and formulations. The key elements of its strategy to achieve this goal include the following:
- Pursuing orphan, genetic epilepsy indications for ganaxolone. Within epilepsy, there are several smaller patient populations, such as CDD, where a genetic marker associated with the syndrome has been linked to deficits in GABAergic signaling. Based on clinical data, the company believe that increasing GABAergic tone with ganaxolone could provide benefit and that treatments for these small populations have the potential for more efficient paths to regulatory approval and commercialization. In addition to CDD, the company may also explore development of ganaxolone in other rare epilepsy indications.
- Broadening dose forms to acute care setting. To date, its clinical trials in patients have utilized its patented nanoparticulate composition administered in oral capsule and liquid suspension dose forms. As a complement to these orally administered dose forms, Marinus Pharmaceuticals has developed an IV dose form for the acute care setting and in-patient populations, such as SE and PPD, that may benefit from inpatient ganaxolone IV before transitioning to an outpatient oral dose form.
- Expanding non‑epilepsy indications for ganaxolone. Due to its mechanism of action, the company believe ganaxolone has potential for therapeutic benefit in a variety of neuropsychiatric disorders in addition to epilepsy. Evidence from preclinical and clinical studies demonstrates that treatment with an agent similar to naturally occurring allo could be of benefit in patients with anxiety, mood, sleep and other neuropsychiatric disorders. The company believe its top-line results from the Phase 2 proof‑of‑concept clinical trials in Fragile X Syndrome (FXS) patients and anecdotal reports from investigators who treated CDD, PCDH19-PE and LGS patients support this hypothesis. Marinus Pharmaceuticals is also exploring development of ganaxolone in PPD, and the company may explore development of ganaxolone in other depression-related, neuropsychiatric disorders and rare neurological diseases.
- Build on its product pipeline. The company intend to expand and diversify its product pipeline through development and/or acquisition of additional drug candidates that fit its business strategy. In addition, the company may expand the targeted indication footprint for its ganaxolone franchise into other epilepsy, neuropsychiatric or other indications.
Intellectual Property
The proprietary nature of and protection for its product candidates, discovery programs and know-how are important to its business. Marinus Pharmaceuticals has sought patent protection in the United States and internationally for ganaxolone synthetic methods and ganaxolone nanoparticles, which are used in oral solid, oral liquid, and intravenous dose formulations, other injectable ganaxolone formulations, and methods of treatment using ganaxolone formulations. The company's policy is to pursue, maintain and defend patent rights whether developed internally or licensed from third parties and to protect the technology, inventions and improvements that are commercially important to the development of its business.
The basis of its intellectual property for ganaxolone nanoparticle formulations was the discovery of a novel composition of ganaxolone nanoparticles and complexing agents that deliver consistent exposure and improved stability of ganaxolone. This discovery resulted in the issuance of its United States and foreign patents, which cover ganaxolone nanoparticle formulations and the use of these formulations for treating seizure disorders. The company's patent portfolio for ganaxolone nanoparticle formulations contains eight United States patents, one pending United States patent application, and corresponding foreign patents and patent applications directed to solid and liquid ganaxolone formulations and methods for the making and use thereof. These patents expire in 2026, excluding accounting for possible patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, or for possible pediatric exclusivity. Corresponding foreign patents have been granted in Australia, Canada, China, Eurasia, India, Israel, Japan, Mexico, South Africa, New Zealand, Singapore and South Korea. Corresponding foreign patent applications are pending in China, Europe and India. Marinus Pharmaceuticals has not licensed any rights to practice these patents in any of these territories. Pursuant to its agreement with Domain Russia Investments Limited, or DRI, the company assigned DRI patent rights, which rights were subsequently assigned to NovaMedica LLC, along with the rights to develop and commercialize ganaxolone in Russia and certain other eastern European nations.
The company's patent portfolio also contains patents issued in Australia, United States, Europe, Japan, Mexico, New Zealand, China, Hong Kong and Israel covering its novel and cost effective ganaxolone synthesis process, which expire in 2030, excluding accounting for possible patent term extension under the Hatch-Waxman Act, or for possible pediatric exclusivity. Corresponding foreign patent applications are pending in Brazil, Canada, India, and South Korea.
The company filed two provisional applications in 2015 directed to intravenous ganaxolone formulations and methods of using these formulations to treat refractory epileptic seizures and other disorders. Both of these patents have been converted to US non-provisional applications with corresponding Patent Cooperation Treaty (PCT) applications. One of these PCT applications has entered the national stage in Australia, Canada, China, Europe, India, Israel, Japan, and South Africa. The company expect to file national stage applications for the other of these PCT applications in 2018. If granted, these patents will expire in 2036, excluding accounting for possible patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 or the Hatch-Waxman Act. The company filed two provisional applications in 2016 directed to additional methods of treatment using its nanoparticulate and IV formulations. In 2017 the company converted one of these applications to a US non-provisional application and converted the other to both a US non-provisional and a PCT application. If subsequently granted, the patents from these applications will expire in 2037. The company filed a provisional application directed to sustained release injectable ganaxolone formulations in 2017. If converted to a non-provisional application and subsequently granted, the patent from this application will expire in 2038.
The company filed a provisional patent application in 2017 directed to the use of its formulations in the treatment of genetic epileptic disorders. The company also filed a provisional patent application in 2017 directed to compositions for the treatment of central nervous system (CNS) disorders. Both patents may be converted to US non-provisional applications with corresponding Patent Cooperation Treaty (PCT) applications in 2018. If converted to non-provisional applications and subsequently granted, the patents from these applications will expire in 2038.
In addition to patents, the company rely upon unpatented trade secrets, know-how and continuing technological innovation to develop and maintain a competitive position. The company seek to protect its proprietary information, in part, through confidentiality agreements with its employees, collaborators, contractors and consultants, and invention assignment agreements with its employees and some of its collaborators. The confidentiality agreements are designed to protect its proprietary information and, in the case of agreements or clauses requiring invention assignment, to grant it ownership of technologies that are developed through a relationship with a third party.
General considerations
As with other biotechnology and pharmaceutical companies, its ability to maintain and solidify a proprietary position for its ganaxolone synthesis and formulations will depend upon its success in obtaining effective patent claims and enforcing those claims once granted. The company's commercial success will depend in part upon not infringing upon the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent could require it to alter its development or commercial strategies, obtain licenses, or cease certain activities. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights.
The term of a patent that covers a FDA-approved drug may be eligible for patent term extension, which provides patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when its pharmaceutical products receive FDA approval, the company expect to apply for patent term extensions on patents covering those products.
Many pharmaceutical companies, biotechnology companies and academic institutions are competing with it in the field of neuropsychiatric disorders and filing patent applications potentially relevant to its business. Even if a particular third-party patent is identified as possibly being relevant to its product candidates or technology, the company may conclude upon a thorough analysis, that the company do not infringe upon the patent or that the patent is invalid. If the third-party patent owner disagrees with its conclusion and the company continue with the business activity in question, the company may be subject to patent litigation. Alternatively, the company might decide to initiate litigation in an attempt to have a court declare the third-party patent invalid or non-infringed by its activity. In either scenario, patent litigation typically is costly and time-consuming, and the outcome can be favorable or unfavorable.
Collaborations
NovaMedica
In connection with its Series C convertible preferred stock financing, in December 2012 the company entered into a Technology Transfer Agreement, or the Transfer Agreement, with DRI, a significant stockholder of its company. Pursuant to the Transfer Agreement, in exchange for a payment of $100,000, the company assigned to DRI certain patents and patents applications in Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Moldova, Russia, Tajikistan, Turkmenistan, Ukraine and Uzbekistan, or the Covered Territory, and granted to DRI an exclusive, royalty-free, irrevocable and assignable license under its know-how to develop and commercialize ganaxolone and other products that would infringe its patent rights or use its know-how, or the Covered Products, in the Covered Territory, in the field of uses for any human or animal disease or condition excluding the treatment of unpleasant sensory or emotiona experience associated with actual or potential tissue damage or described in terms of such damage, or the Field. Immediately thereafter, we, together with DRI, executed an Assignment and Assumption Agreement, pursuant to which all of DRI’s rights and obligations under the Transfer Agreement were transferred to NovaMedica, LLC, or NovaMedica. The company agreed to take all action required to register or record the patent transfers to DRI in each country in the Covered Territory and to ensure the assignment of DRI’s rights under the Transfer Agreement to NovaMedica. NovaMedica is jointly owned by Rusnano Medinvest LLC, or Rusnano Medinvest, and DRI. RMI Investments, S.á.r.l, a stockholder of ours, is a wholly-owned subsidiary of Rusnano Medinvest.
Under the terms of the Transfer Agreement, NovaMedica, or its permitted transferees or assignees, has the exclusive right within the Covered Territory to manufacture the Covered Products solely for development and commercialization in the Covered Territory in the Field. Until the first commercial sale of a Covered Product within the Covered Territory, NovaMedica will have the right to purchase supplies of the Covered Product from it as are reasonably available to it and as are reasonable and necessary to conduct clinical trials of Covered Product in the Covered Territory, provided that any such purchase does not reasonably interfere with its having sufficient supplies of Covered Products on hand for use in development (including the conduct of clinical trials) or commercialization outside of the Covered Territory. Such purchases will be made on a cost-plus basis. The Transfer Agreement provides that the parties shall enter into the Supply Agreement to supply ganaxolone and/or Covered Product for development in the Covered Territory within 60 calendar days from NovaMedica’s request, which Marinus Pharmaceuticals has not yet received.
In accordance with the terms of the Transfer Agreement, on June 25, 2013 the company entered into a Clinical Development and Collaboration Agreement, or the Collaboration Agreement, with NovaMedica, pursuant to which the company agreed to assist NovaMedica in the development and commercialization of Covered Products in the Covered Territory in the Field. The Collaboration Agreement requires the formation of committees consisting of its representatives and NovaMedica representatives to oversee the general development, day-to-day development work and commercialization of Covered Products in the Field in the Covered Territory. All decisions of these committees must be made by unanimous vote, subject to a dispute resolution process. Under the terms of the Collaboration Agreement, the joint committees will determine a development plan for ganaxolone in clinical trials and a plan for commercialization of ganaxolone. NovaMedica will have sole responsibility for the costs and expenses of obtaining regulatory approval for Covered Products and for commercializing any approved products in the Covered Territory, and NovaMedica will have the right to conduct its own clinical studies in the Covered Territory at its sole expense. NovaMedica also has the right to file applications for approval of Covered Products in the Covered Territory, subject to committee oversight. Marinus Pharmaceuticals has agreed, among other things, to provide NovaMedica with data and regulatory files necessary for it to obtain necessary approvals in the Covered Territory, information relating to applications for regulatory approval of Covered Products, certain commercialization information and to assist NovaMedica in conducting any clinical trials necessary for regulatory approval of Covered Products in the Covered Territory. The company also have agreed to provide NovaMedica with certain development know-how and support, including making its clinical development personnel available to provide scientific and technical explanations, consultation and support that may be reasonably requested by NovaMedica.
NovaMedica is required to reimburse it for any out-of-pocket expenses incurred by it in providing this assistance, except for expenses incurred in its participation on the joint committees. Pursuant to the Collaboration Agreement and the Transfer Agreement, Marinus Pharmaceuticals has agreed to use commercially reasonable efforts to include sites in the Russian Federation in its clinical trial programs for the first indications of the Covered Products at its sole expense. Under the Transfer Agreement, at least 36 months prior to the first commercial sale of a product candidate in the Covered Territory, the parties have agreed to negotiate in good faith a supply agreement pursuant to which the company or a third party contract manufacturer authorized by it to manufacture and supply the Covered Products, will supply needed quantities of Covered Product to NovaMedica solely for commercialization of Covered Products in the Covered Territory, on commercially fair and reasonable terms. Such purchases will be made on a cost-plus basis. In the event the parties are unable to agree on pricing under the supply agreement, they have agreed to engage an internationally recognized consulting firm reasonably acceptable to both parties to perform an analysis to determine final pricing under the supply agreement, which decision will be binding upon the parties. In the event that the parties are unable to reach a reasonably acceptable supply agreement or Marinus Pharmaceuticals is unable to supply Covered Products to NovaMedica under such supply agreement for a period of at least 60 calendar days after the specified delivery date and the company thereafter fail to cure such failure within 60 days after written notice from NovaMedica, Marinus Pharmaceuticals has agreed to cooperate with NovaMedica to identify a mutually acceptable alternative source of supply and will provide the necessary consents to allow such alternative source of supply to provide the needed quantities of the Covered Products to NovaMedica. The terms of the alternative source of supply would be negotiated directly by NovaMedica with the supplier.
The Collaboration Agreement expires on the earlier of three years following the first commercial sale of a product candidate in the Covered Territory or the termination of the Transfer Agreement. NovaMedica also has the right to terminate the Collaboration Agreement at any time at its convenience upon 90 days’ prior written notice.
Purdue Neuroscience Company (Purdue)
In September 2004, the company entered into a license agreement with Purdue, which was most recently amended and restated in May 2008, that granted it exclusive rights to certain know-how and technology relating to ganaxolone, excluding the field of treatment of unpleasant sensory or emotional experience associated with actual or potential tissue damage or described in terms of such damage. The agreement contains a right by it to sublicense subject to prior written approval by Purdue and Marinus Pharmaceuticals has sublicensed its licensed rights to NovaMedica for the Covered Territory. Marinus Pharmaceuticals is obligated to pay royalties as a percentage in the range of high single digits up to 10% of net product sales for direct licensed products, such as ganaxolone. The obligation to pay royalties expires, on a country-by-country basis, ten years from the first commercial sale of a licensed product in each country. Upon commercialization, the company estimate the in‑licensed technology would result in its paying royalties to Purdue in the low single digits as a percentage of sales. Other payment obligations may be triggered if the company successfully partner its product candidates with third parties. In addition, the agreement also requires that the company pay Purdue a percentage in the mid-single digits of the non-royalty consideration that the company receive from a sublicensee and a percentage in the twenties of milestone payments received from sublicensees for indications other than seizure disorders and vascular migraine headaches not associated with mood disorders. Under the license agreement, Marinus Pharmaceuticals is committed to use commercially reasonable efforts to develop and commercialize at least one licensed product.
Competitive Landscape
The company primarily compete with pharmaceutical and biotechnology companies that are developing therapies or marketing drugs to treat indications that Marinus Pharmaceuticals is targeting.
CDD
There are no drugs approved for the treatment of CDD. CDD patients are typically prescribed drugs approved for epileptic seizures, which often fail to control seizures in this patient population. To its knowledge, there is one other company currently conducting a Phase 2 clinical trial in CDD patients.
PPD
Approximately 500,000-750,000 mothers suffer from postpartum depression annually in the US. The majority of women suffering from depression do not seek treatment. There are no approved treatments for PPD, however the most common treatments are psychotherapy and prescription antidepressants. Many women who take antidepressants discontinue them prior to and after parturition due to concern for the child. Sage Therapeutics is developing an intravenous formulation of allo and a new orally-administered chemical entity for PPD.
SE
SE patients generally are treated with benzodiazepine as first-line treatment. When benzodiazepines are not effective, several AEDs are used. When second-line AEDs are not effective, the patient is generally placed under IV anesthesia as a last resort to attempt to stop the seizures and prevent further damage to the brain and death. Morbidity and mortality rates increase for patients that progress to SRSE. To its knowledge, there are no other companies currently conducting clinical studies in SE patients.
Manufacturing
Manufacturing of drugs and product candidates, including ganaxolone, must comply with FDA current good manufacturing practice, or cGMP, regulations. Ganaxolone is a synthetic small molecule made through a series of organic chemistry steps starting with commercially available organic chemical raw materials. The company conduct manufacturing activities under individual purchase orders with independent contract manufacturing organizations, or CMOs, to supply its clinical trials. Marinus Pharmaceuticals has an internal quality program and have qualified and signed quality agreements with its major CMOs. The company conduct periodic quality audits of their facilities. The company believe that its existing suppliers of ganaxolone’s active pharmaceutical ingredient and finished product will be capable of providing sufficient quantities of each to meet its clinical trial supply needs. Other CMOs may be used in the future for clinical supplies and, subject to approval, commercial manufacturing.
Ganaxolone Formulations
The therapeutic possibilities of ganaxolone have been understood for some time, however, because ganaxolone is a high-dose water insoluble compound, developing a formulation that could provide consistent drug exposure and could be manufactured at a commercially feasible cost had proven challenging. The company believe its patented nanoparticulate formulation and novel manufacturing process for ganaxolone can successfully address the cost of manufacturing and pharmacokinetic challenges that previously encumbered the clinical and commercial feasibility of ganaxolone.
Ganaxolone is currently formulated as an IV, liquid suspension and as a capsule.
Commercial Operations
If the company obtain FDA approval for ganaxolone, the company intend to build a sales and marketing infrastructure to reach high prescribing neurologist, critical care, epilepsy specialists and other target physician populations in the United States. The company believe a focused sales and marketing organization could be leveraged to market ganaxolone across multiple epilepsy, neurology or psychiatry indications if Marinus Pharmaceuticals is able to obtain regulatory approval for those other indications. The company may seek co-promotion partners for its sales efforts to reach other United States physician groups, such as primary care physicians. The company believe that there could also be significant market opportunities for ganaxolone in epilepsy and other neurological and psychiatric conditions outside of the United States. In order to capitalize on such opportunities, the company plan to seek collaborations with pharmaceutical companies that have greater reach and resources by virtue of their size and experience in the field.




