Palatin Technologies
Overview
Palatin Technologie (PTN) is a biopharmaceutical company developing targeted, receptor-specific peptide therapeutics for the treatment of diseases with significant unmet medical need and commercial potential. Its programs are based on molecules that modulate the activity of the melanocortin and natriuretic peptide receptor systems. Its lead product in clinical development is bremelanotide for the treatment of premenopausal women with hypoactive sexual desire disorder (“HSDD”), which is a type of female sexual dysfunction (“FSD”), defined as low desire with associated distress. In addition, Palatin has drug candidates and development programs for cardiovascular diseases and inflammatory diseases.1
The following drug development programs are actively under development:
- Bremelanotide, an as-needed subcutaneous injectable product for the treatment of HSDD in premenopausal women. Bremelanotide is a synthetic peptide analog of the naturally occurring hormone alpha-MSH (melanocyte-stimulating hormone). In two pivotal Phase 3 clinical studies of bremelanotide for HSDD in premenopausal women, bremelanotide met the pre-specified co-primary efficacy endpoints of improvement in desire and decrease in distress associated with low sexual desire as measured using validated patient-reported outcome instruments. Palatin has licensed North American rights to bremelanotide to AMAG Pharmaceuticals, Inc. (“AMAG”), and rights in China, Taiwan, Hong Kong and Macau to Shanghai Fosun Pharmaceutical Industrial Development Co., Ltd. (“Fosun”);
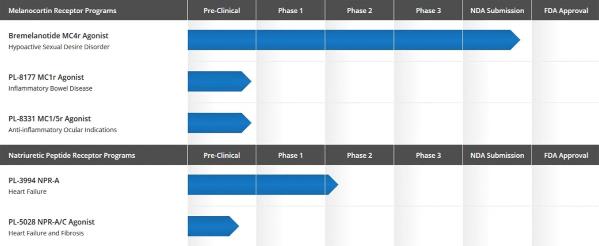
- Melanocortin peptide system program, focused on development of treatments for a variety of inflammatory disease indications. PL-8177 is a selective melanocortin receptor 1 (“MC1r”) agonist peptide Palatin has designated as its lead clinical development candidate for inflammatory bowel diseases. Palatin is scheduled to file an IND application this year, and may thereafter initiate a Phase 1 clinical safety study. A dual melanocortin receptor 1 and 5 peptide the company developed, PL-8331, is a preclinical development candidate for treating ocular inflammation. The company anticipate completing preclinical IND enabling activities on PL-8331 this calendar year; and
- Natriuretic peptide system program, including PL3994, a natriuretic peptide receptor-A (“NPR-A”) agonist, for treatment of cardiovascular indications. PL3994, a synthetic mimetic of the neuropeptide hormone atrial natriuretic peptide (“ANP”), is in development for treatment of heart failure, and is scheduled to start Phase 2A clinical trials later this calendar year. A dual natriuretic peptide receptor A and C agonist the company developed, PL-5028, is in preclinical development for cardiovascular diseases, including reducing cardiac hypertrophy and fibrosis. The company may file an Investigational New Drug (“IND”), application in the first half of calendar year 2018, and thereafter initiate a Phase 1 clinical safety study.
The following chart illustrates the status of its drug development programs.
Strategy
Key elements of its business strategy include:
Using its technology and expertise to develop and commercialize products in its active drug development programs; Entering into strategic alliances and partnerships with pharmaceutical companies to facilitate the development, manufacture, marketing, sale and distribution of its product candidates; Partially funding its product development programs with the cash flow generated from existing license agreements, as well as any potential future research, collaboration or license agreements with third parties; and Completing development and seeking regulatory approval of certain of its product candidates.
Melanocortin Receptor-Specific Programs
The melanocortin system is involved in a large and diverse number of physiologic functions. Therapeutic agents modulating this system may have the potential to treat a variety of conditions and diseases, including sexual dysfunction, obesity and related disorders, pigmentation disorders and inflammation-related diseases.
Bremelanotide for HSDD. Palatin is developing subcutaneously administered bremelanotide for the treatment of HSDD in premenopausal women. HSDD is characterized by both a decrease in sexual desire and significant personal distress or interpersonal difficulty as a result of the lack of desire. Bremelanotide is a melanocortin agonist with a mechanism of action which the company believe involves activation of endogenous neuronal pathways in the brain regulating sexual arousal and desire responses.
The company completed last patient visits in the efficacy parts of its two pivotal Phase 3 clinical studies of bremelanotide for the treatment of HSDD in premenopausal women in the third quarter of calendar year 2016. The company announced topline efficacy results in the fourth quarter of calendar year 2016. The open-label safety extension portions of its pivotal Phase 3 clinical studies were completed in the second quarter of calendar year 2017.
Palatin's Phase 3 clinical study program consisted of two randomized, double-blinded, placebo-controlled Phase 3 studies, Studies 301 and 302, comparing the efficacy and safety of bremelanotide versus placebo in premenopausal women diagnosed with HSDD. The primary efficacy analysis population was the modified intent-to-treat patient population, consisting of 1,202 women with HSDD in the United States and Canada. Patients self-administered either 1.75 mg of bremelanotide or placebo as needed in anticipation of sexual activity. The efficacy portion of each study consisted of a 24-week treatment evaluation period.
Based on discussions with the U.S. Food and Drug Administration (“FDA”), it was decided that the co-primary endpoints for the Phase 3 clinical trials were the Female Sexual Function Index: Desire Domain (“FSFI-D”) and Female Sexual Distress Scale-Desires/Arousal/Orgasm (“FSDS-DAO”) Item 13. The FSFI-D is a validated patient reported outcome measurement tool of sexual desire in the context of overall sexual function. The FSDS-DAO Item 13 is a validated patient reported outcome measurement tool of distress related to sexual dysfunction, measuring personal distress associated with low sexual desire. Both Phase 3 Studies 301 and 302 with bremelanotide for HSDD in premenopausal women met the pre-specified co-primary efficacy endpoints.
The FSFI-D showed a statistically significant increase for bremelanotide compared to placebo in both trials in the modified intent-to-treat patient population:
Study 301: Mean change of 0.54 vs. 0.24, median change of 0.60 vs. 0.00, p=0.0002; and,
Study 302: Mean change of 0.63 vs. 0.21, median change of 0.60 vs. 0.00, p<0.0001.
The FSDS-DAO Item 13 showed a statistically significant reduction in distress related to low sexual desire for bremelanotide compared to placebo in both trials in the modified intent-to-treat patient population:
Study 301: Mean change of -0.73 vs. -0.36, median change of -1.0 vs. 0.0, p<0.0001; and,
Study 302: Mean change of -0.71 vs. -0.42, median change of -1.0 vs. 0.0, p=0.0053.
The changes seen in both co-primary endpoints were clinically significant. An independent committee evaluated the clinical significance of co-primary endpoint study results using multiple assessments of patient benefit, and was based on discussions with the FDA and FDA guidance documents.
In the safety population (1,247 patients), bremelanotide appeared to be well tolerated. The most frequent adverse event was nausea, which was generally mild in nature. The safety profile of bremelanotide was consistent with prior clinical experience.
In the Phase 3 clinical study program patients self-administered bremelanotide with a single-use autoinjector pen. The bremelanotide single-use autoinjector pen, intended to be the commercial drug product, does not have a visible needle, is stored at room temperature and is easy to use. Women administer bremelanotide by pressing the autoinjector pen collar against either their thigh or abdomen, and the autoinjector pen automatically introduces the needle, administers the dose of bremelanotide under the skin and audibly signals when the drug had been delivered and the needle has been retracted.
Ongoing Studies and New Drug Application. Palatin is conducting multiple pharmacokinetic and safety pharmacology studies, including an abuse-liability study and drug-to-drug interaction studies, as well as certain chemistry, manufacturing and controls activities, including a drug product process validation study. The company anticipate that the required human clinical studies will be completed this calendar year. The company currently expect that the company will, with AMAG, its North American licensee of bremelanotide, submit a New Drug Application (“NDA”) to FDA for bremelanotide for the treatment of HSDD in early calendar year 2018 following completion of ongoing studies. The company cannot assure you that a complete review of the Phase 3 efficacy data and the pharmacokinetic and safety pharmacology studies will support approval of bremelanotide for HSDD or that the FDA will approve an NDA for bremelanotide.
Medical Need — HSDD. HSDD, either with or without arousal difficulties, is the largest single category of FSD. FSD is a multifactorial condition that has anatomical, physiological, medical, psychological and social components, and is defined as persistent or recurring problems during one or more of the stages of sexual response with associated distress. HSDD has a significant impact on a patient’s self-image, relationships and general well-being. The 2006 PRESIDE (Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking) study, a cross-sectional, population-based survey of 31,581 female adult respondents in the United States published in 2008 in the journal Obstetrics & Gynecology, found that approximately 22% of women reported a sexual problem and 11% were women with HSDD. Based on the number of premenopausal women in the United States according to the U.S. Census, the presenting market size of premenopausal women with primary HSDD is at least 5.8 million women.
Subcutaneous Bremelanotide. Bremelanotide, which is believed to act through activation of melanocortin receptors in the central nervous system, is a first-in-class pharmaceutical agent in development as a treatment of HSDD. Bremelanotide is intended for as needed use and is self-administered by the patient, using a simple and patient-friendly single-use autoinjector pen, thirty minutes to one hour prior to anticipated sexual activity.
Partnering. In January 2017, the company entered into a license agreement with AMAG, pursuant to which the company granted AMAG an exclusive license in all countries of North America, with the right to grant sublicenses, to research, develop and commercialize products containing bremelanotide. AMAG also has a non-exclusive license, with the right to grant sublicenses, to manufacture products containing bremelanotide in North America, and to research, develop and manufacture, but not commercialize, products containing bremelanotide in countries outside North America. Upon the license agreement becoming effective on February 2, 2017, AMAG paid it $60 million as a one-time initial payment, and is required to pay it up to $25 million to reimburse it for direct out-of-pocket expenses incurred in development and regulatory activities necessary to file an NDA. In addition, the company may receive up to $80 million in specified regulatory payments upon achievement of certain regulatory milestones, and up to $300 million in sales milestone payments based on achievement of certain annual net sales amounts of products containing bremelanotide. AMAG will also pay tiered royalties on annual net sales of products containing bremelanotide at rates ranging from the high single-digits to the low double-digits.
In early September 2017, the company entered into a license agreement with Fosun for exclusive rights to commercialize bremelanotide in the territories of mainland China, Taiwan, Hong Kong S.A.R. and Macau S.A.R. The company will receive an upfront payment of $5.0 million and, when regulatory approval for a bremelanotide product is obtained in China, a $7.5 million milestone payment. The company may receive up to $92.5 million in sales related milestones, and will receive high single-digit to low double-digit royalties on net sales in the licensed territories.
The company retain worldwide rights for bremelanotide for FSD, HSDD and all other indications outside North America and the territories licensed to Fosun. Palatin is in active discussions with potential partners for marketing and commercialization rights for bremelanotide in other jurisdictions, including Europe. The company may not be able to enter into suitable agreements with potential partners on acceptable terms, if at all.
Prior Clinical Trials. Palatin has completed several Phase 1 clinical studies in which various safety parameters, including blood pressure effects of subcutaneously administered bremelanotide, were studied. Based in part on these studies, its Phase 2B clinical trial assessed the magnitude and duration of blood pressure effect, and determined that subcutaneous administration of selected doses of bremelanotide for treatment of HSDD in premenopausal women provides acceptable control of blood pressure effects.
MC1r Peptide Agonists. Palatin has conducted preclinical animal studies with MC1r peptide drug candidates for a number of inflammatory disease and autoimmune indications. The MC1r is upregulated in a number of diseases, including inflammatory bowel disease, nephritis, which is inflammation of the kidneys, and rheumatoid arthritis, and in ocular indications such as uveitis and dry eye. The company believe that MC1r peptides have broad anti-inflammatory effects and appear to utilize mechanisms engaged by the endogenous melanocortin system in regulation of the immune system and resolution of pro-inflammatory responses.
Palatin's MC1r peptide drug candidates are highly specific, with substantially greater binding and activity at MC1r than at other melanocortin receptors. In vitro safety studies have shown that its MC1r peptide drug candidates have no activity in a wide range of receptors, ion channels and kinases. Palatin has selected one of its MC1r peptide drug candidates, designated PL-8177, as a clinical trial candidate. PL-8177 is a selective MC1r agonist peptide Palatin has designated as its lead clinical development candidate for inflammatory bowel diseases. Palatin has completed preclinical toxicology testing on PL-8177 and chemistry, controls and manufacturing activities to support Phase 1 studies, and anticipate filing an IND application on PL-8177 this calendar year, and may thereafter to initiate Phase 1 clinical safety studies.
Palatin is also developing a peptide which is a dual melanocortin receptor 1 and 5 agonist, PL-8331, which is a preclinical development candidate for treating ocular inflammatory diseases. The company anticipate completing preclinical IND enabling activities with PL-8331 by the first half of calendar year 2018.
Next Generation Melanocortin Receptor 4 (“MC4r”) Peptide and Small Molecule Agonists. Palatin has developed a series of highly selective MC4r peptides and orally active small molecules. In developing these compounds, the company examined effectiveness in animal models of sexual response, obesity and related metabolic signals, and also determined cardiovascular effects, primarily looking at changes in blood pressure. Results of these studies suggest that certain of these compounds may have significant medical and commercial potential for treatment of conditions responsive to MC4r activation, including HSDD, FSD, ED, obesity and diabetes. Palatin is seeking collaboration and development partners for these compounds for obesity and related clinical indications, but may not be able to enter into suitable agreements on acceptable terms with potential partners, if at all.
Natriuretic Peptide Receptor-Specific Programs
The natriuretic peptide receptor system has numerous cardiovascular functions, and therapeutic agents modulating this system may be useful in treatment of heart failure, acute asthma, other pulmonary diseases and hypertension. While the therapeutic potential of modulating this system is well appreciated, development of therapeutic agents has been difficult due, in part, to the short biological half-life of native peptide agonists.
Palatin has designed and are developing potential candidate drugs that are selective for different natriuretic peptide receptors, including NPR-A, natriuretic peptide receptor B (“NPR-B”), natriuretic peptide receptor C, and both NPR-A and NPR-B.
PL-3994. PL-3994 is its lead natriuretic peptide receptor product candidate, and is a synthetic mimetic of the neuropeptide hormone ANP and an NPR-A agonist. PL-3994 is in development for treatment of heart failure (with preserved or reduced ejection fraction) and may be suitable for replacement therapy in patients with prohormone processing deficiencies. PL-3994 activates NPR-A, a receptor known to play a role in cardiovascular homeostasis. Consistent with being an NPR-A agonist, PL-3994 increases plasma cyclic guanosine monophosphate (“cGMP”), levels, a pharmacological response consistent with the effects of endogenous (naturally produced) natriuretic peptides on cardiovascular function and smooth muscle relaxation. PL-3994 also decreases activity of the renin-angiotensin-aldosterone system (“RAAS”), a hormone system that regulates blood pressure and fluid balance. The RAAS system is frequently over-activated in heart failure patients, leading to worsening of cardiovascular function.
PL-3994, its lead product development candidate which is ready for Phase 2 safety and efficacy studies, is one of a number of natriuretic peptide receptor agonist compounds Palatin has developed. In conjunction with clinicians at a major research institution, PL-3994 is scheduled to enter Phase 2A clinical trials later in calendar year 2017. PL-3994 is a synthetic molecule incorporating a novel and proprietary amino acid mimetic structure, and has an extended circulation half-life and metabolic stability compared to endogenous ANP. Based on the half-life and pharmacokinetics, the company believe that PL-3994 is amenable to once daily chronic use subcutaneous administration.
Prior Clinical Studies with PL-3994. Human clinical studies of PL-3994 commenced with a Phase 1 trial, which concluded in 2008. This was a randomized, double-blind, placebo-controlled study in 26 healthy volunteers who received either PL-3994 or a placebo subcutaneously. Dosing concluded with the successful achievement of the primary endpoint of the study, a pre-specified reduction in systemic blood pressure. No volunteer experienced a serious or severe adverse event. Elevations in plasma cGMP levels, increased diuresis and increased natriuresis were all observed for several hours after single subcutaneous doses. Later in 2008, the company conducted a trial in volunteers with controlled hypertension who were receiving one or more conventional antihypertensive medications. No volunteer experienced a serious or severe adverse event. Elevations in plasma cGMP levels were observed for several hours after single subcutaneous doses. Based on the studies to date, PL-3994 is ready for Phase 2 safety and efficacy studies.
PL-5028. Palatin is in preclinical development with PL-5028, a dual natriuretic peptide receptor A and C agonist the company developed, for cardiovascular disease indications, including reducing cardiac hypertrophy and fibrosis. The company may file an IND application in the first half of calendar year 2018, and thereafter initiate a Phase 1 clinical safety study.
Administration of PL-3994 and PL-5028. For heart failure and other cardiovascular disease indications the company believe that subcutaneous administration may be employed. In studies to date, PL-3994 is well absorbed through the subcutaneous route of administration. In human studies with PL-3994, the pharmacokinetic and pharmacodynamic half-lives were on the order of hours, significantly longer than the comparable half-lives of endogenous natriuretic peptides. The company believe that subcutaneous PL-3994 or PL-5028, if successful, will be appropriate for self-administration by patients, similar to insulin and other self-administered drugs.
Heart Failure. Heart failure is an illness in which the heart is unable to pump blood efficiently, and includes acutely decompensated heart failure with dyspnea (shortness of breath) at rest or with minimal activity. Endogenous natriuretic peptides have a number of beneficial effects, including vasodilation (relaxation of blood vessels), natriuresis (excretion of sodium) and diuresis (excretion of fluids).
Patients who have been admitted to the hospital with an episode of worsening heart failure have an increased risk of either death or hospital readmission in the three months following discharge. Up to 15% of patients die in this period and as many as 30% need to be readmitted to the hospital. The company believe that decreasing mortality and hospital readmission in patients discharged following hospitalization for worsening heart failure is a large unmet medical need for which PL-3994 may be effective. PL-3994 could potentially be utilized as an adjunct to existing heart failure medications, and may, if successfully developed, be self-administered by patients as a subcutaneous injection following hospital discharge. The company believe that its natriuretic peptide products under development may, if successful, reduce cardiac hypertrophy (increase in heart size due to disease), which is an independent risk factor for cardiovascular morbidity and mortality.
According to a report from the American Heart Association published in 2014 in the journal Circulation, an estimated 5.7 million Americans suffer from heart failure, with 870,000 new cases of heart failure diagnosed each year, with disease incidence expected to increase with the aging of the American population. Heart failure has tremendous human and financial costs. The same report estimated that the 2012 total costs in the United States for heart failure were $30.7 billion, with heart failure constituting the leading cause of hospitalization in people over 65 years of age and with over 1 million hospital discharges for heart failure in 2010. Heart failure is a high mortality disease, with approximately one-half of heart failure patients dying within five years of initial diagnosis.
Patient populations have been identified which have reduced levels of endogenous active natriuretic peptides, including endogenous active ANP. The reduced levels have a variety of causes, including mutations in endogenous natriuretic peptides and in enzymes necessary to convert natriuretic peptide sequences to their active form. Patients with reduced levels of endogenous active natriuretic peptides are reported to have a poor response to current drug therapies and to have increased rates of cardiac remodeling and cardiac events.
The company believe that PL-3994 has the potential to treat heart failure with preserved ejection fraction (“HFpEF”), which is a high unmet medical need with no approved treatment options, heart failure with reduced ejection fraction (“HFrEF”), and patients with reduced levels of endogenous active natriuretic peptides, such as corin deficiencies, which is a high unmet medical need in patients with a poor response to current therapies, with the objective to restore normal natriuretic peptide function.
Technologies Use
The company used a rational drug design approach to discover and develop proprietary peptide, peptide mimetic and small molecule agonist compounds, focusing on melanocortin and natriuretic peptide receptor systems. Computer-aided drug design models of receptors are optimized based on experimental results obtained with peptides and small molecules that the company develop. With its approach, the company believe Palatin is developing an advanced understanding of the factors which drive agonism.
Palatin has developed a series of proprietary technologies used in its drug development programs. One technology employs novel amino acid mimetics in place of selected amino acids. These mimetics provide the receptor-binding functions of conventional amino acids while providing structural, functional and physiochemical advantages. The amino acid mimetic technology is employed in PL-3994, its compound in development for treatment of heart failure.
Some compound series have been derived using its proprietary and patented platform technology, called MIDAS™, or Metal Ion-induced Distinctive Array of Structures. This technology employs metal ions to fix the three-dimensional configuration of peptides, forming conformationally rigid molecules that remain folded specifically in their active state. These MIDAS molecules are generally simple to synthesize, are chemically and proteolytically stable, and have the potential to be orally bioavailable. In addition, MIDAS molecules are information-rich and provide data on structure-activity relationships that may be used to design small molecule, non-peptide drugs.
Amount Spent on Research and Development Activities
Research and development expenses were approximately $45.7 million for the fiscal year ended June 30, 2017 (“ fiscal 2017”), $43.1 million for the fiscal year ended June 30, 2016 (“fiscal 2016”), and $24.6 million for the fiscal year ended June 30, 2015 (“fiscal 2015”).
Competition
General. Its products under development will compete on the basis of quality, performance, cost effectiveness and application suitability with numerous established products and technologies. Palatin has many competitors, including pharmaceutical, biopharmaceutical and biotechnology companies. Furthermore, there are several well-established products in its target markets that the company will have to compete against. Products using new technologies which may be competitive with its proposed products may also be introduced by others. Most of the companies selling or developing competitive products have financial, technological, manufacturing and distribution resources significantly greater than its and may represent significant competition for it. In addition, if any of its product candidates are approved by FDA, they will eventually face competition from generic versions that will sell at significantly reduced prices, be preferred by managed care and health insurance payers, and be eligible for automatic pharmacy substitution even when a prescriber writes a prescription for its product. The timing and extent of future generic competition is dependent upon both its intellectual property rights and the FDA regulatory process, but cannot be accurately predicted.
The pharmaceutical and biotechnology industries are characterized by extensive research efforts and rapid technological change. Many biopharmaceutical companies have developed or are working to develop products similar to its or that address the same markets. Such companies may succeed in developing technologies and products that are more effective or less costly than any of those that the company may develop. Such companies may be more successful than it in developing, manufacturing and marketing products.
The company cannot guarantee that the company will be able to compete successfully in the future or that developments by others will not render its proposed products under development or any future product candidates obsolete or noncompetitive or that its collaborators or customers will not choose to use competing technologies or products.
Bremelanotide for Treatment of HSDD. There is competition and financial incentive to develop, market and sell drugs for the treatment of HSDD and other forms of FSD. Flibanserin, sold under the trade name Addyi®, is the only drug currently approved in the United States for treatment of HSDD. Flibanserin, a non-hormonal oral serotonin 5-HT1A agonist, 5-HT2A antagonist, which requires chronic dosing, was approved by the FDA on August 18, 2015 for treatment of premenopausal women with HSDD. The FDA approval included a risk evaluation and mitigation strategy (“REMS”) because of the increased risk of severe hypotension and syncope due to the interaction between flibanserin and alcohol, and a Boxed Warning to highlight the risks of severe hypotension and syncope in patients who drink alcohol during treatment with flibanserin, in those who also use moderate or strong CYP3A4 inhibitors, and in those who have liver impairment. Palatin is aware of several other drugs at various stages of development, most of which are taken on a chronic, typically once-daily, basis. There are other companies reported to be developing new drugs for FSD indications, some of which may be in clinical trials in the United States or elsewhere. Palatin is not aware of any company actively developing a melanocortin receptor agonist drug for HSDD.
PL-3994 and PL-5028 for Heart Failure Indications. Nesiritide (sold under the trade name Natrecor®), a recombinant human B-type natriuretic peptide drug, is marketed in the United States by Scios Inc., a Johnson & Johnson company. Nesiritide is approved for treatment of acutely decompensated congestive heart failure patients who have dyspnea at rest or with minimal activity. Other peptide drugs, including carperitide, a recombinant human ANP drug, and ularitide, a synthetic form of urodilatin, a naturally occurring human natriuretic peptide related to ANP, have been investigated for treatment of congestive heart failure, but Palatin is not aware of any active development in the United States. Palatin is aware of other companies developing intravenously administered natriuretic peptide drugs, with at least one reported to have completed Phase 2 clinical trials for acute heart failure. A combination drug comprised of sacubitril and valsartan developed by Novartis AG, sold under the trade name Entresto®, inhibits both the angiotensin II receptor and neprilysin (an enzyme which inactivates endogenous active natriuretic peptides). This combination drug, which was approved by the FDA in July 2015, results in increases of endogenous active ANP levels, and thus has a mechanism of action with similarities to PL-3994 and PL-5028. In a Phase 3 trial, the combination drug was compared to an angiotensin-converting-enzyme inhibitor, enalapril, in heart failure patients with reduced ejection fraction. It significantly improved the rate of death from cardiovascular causes, significantly reduced hospitalization for heart failure and significantly improved heart failure symptoms. This combination drug demonstrated that upregulation of the natriuretic peptide system in combination with angiotensin-converting-enzyme inhibition is superior to angiotensin-converting-enzyme inhibition alone, and thus provides validation of the natriuretic peptide system as a target for improving outcomes in treating heart failure patients. In addition, there are a number of approved drugs and drugs in development for treatment of heart failure through mechanisms or pathways other than agonism of NPR-A.
MC1r Peptides for Inflammatory Disease-Related Indications. Many inflammatory disease-related indications are treated using systemic steroids or other immunosuppressant drugs, all of which have side effects which can be dose limiting. There are a large number of approved biological drugs and biological drugs under development for treatment of inflammatory disease-related indications. For inflammatory bowel diseases, FDA-approved drugs include mesalazine and immunosuppressive drugs such as prednisone and other steroids, tumor necrosis factor inhibitors such as infliximab and adalimumab, and immune system suppressants such as azathioprine, mercaptopurine and methotrexate.
Obesity. There are a number of FDA-approved drugs and medical devices for the treatment of obesity, and a large number of products in clinical development by other companies, including products which target melanocortin receptors. At least one Phase 2 study has been reported on use of an MC4r agonist for obesity indications.
Patents and Proprietary Information
Patent Protection. Its success will depend in substantial part on its ability to obtain, defend and enforce patents, maintain trade secrets and operate without infringing upon the proprietary rights of others, both in the United States and abroad. The company own a number of issued United States patents and have pending United States patent applications, many with issued or pending counterpart patents in selected foreign countries. The company seek patent protection for its technologies and products in the United States and those foreign countries where the company believe patent protection is commercially important.
The company own two issued United States patents claiming the bremelanotide substance and an issued patent claiming the bremelanotide substance in each of Australia, Austria, Belgium, Brazil, Canada, Cyprus, Denmark, Finland, France, Germany, Greece, Hong Kong, Ireland, Italy, Japan, Korea, Luxembourg, Mexico, Monaco, Netherlands, New Zealand, Portugal, Spain, Sweden, Switzerland, and the United Kingdom. The issued United States patents have a term until 2020, which term may be subject to extension for a maximum period of up to five years as compensation for patent term lost during drug development and the FDA regulatory review process, pursuant to the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments. Whether the company will be able to obtain patent term extensions under the Hatch-Waxman Amendments and the length of any such extension cannot be determined until the FDA approves for marketing, if ever, a product in which bremelanotide is the active ingredient. In addition, the claims of issued patents covering bremelanotide may not provide meaningful protection. Further, third parties may challenge the validity or scope of any issued patent, and under the Hatch-Waxman Amendments, potentially receive approval of a competing generic version of its product or products even before a court rules on the validity or infringement of its patents.
The company own two issued United States patents and pending patent applications in the United States for methods of treating FSD with bremelanotide, and related patent applications are pending in Australia, Brazil, Canada, China, Georgia, Hong Kong, India, Indonesia, Israel, Japan, Korea, Malaysia, Mexico, New Zealand, Philippines, South Africa, Ukraine, Vietnam and before the European and Eurasian patent offices. The issued United States patent has a term until 2033. Whether the company will be able to obtain a patent term extension in the United States under the Hatch-Waxman Amendments, assuming that a relevant patent issues in the United States, and the length of any such extension, cannot be determined until the FDA approves for marketing, if ever, a product utilizing bremelanotide by methods claimed in the patent. Issued patents and pending applications in the United States and elsewhere in the world have a presumptive term, if a patent is issued, until 2033.
Palatin has patents and patent applications on an alternative class of melanocortin receptor-specific peptides for treatment of sexual dysfunction and other indications, including obesity, consisting of two issued patents in the United States, an issued patent in each of Australia, Canada, China, France, Germany, Ireland, Israel, Japan, Korea, Mexico, New Zealand, Russia, Switzerland and the United Kingdom, and pending patent applications on the same class in Brazil, India, and South Africa. The presumptive term of the issued patents and pending patent applications is until 2029. The company also have patents and pending patent applications for a second class of alternative melanocortin receptor-specific peptides for treatment of sexual dysfunction and other indications, including obesity, consisting of issued patents in the United States, Australia, China, Japan, Israel, Korea, New Zealand, Russia, and South Africa and pending patent applications on the same class in Brazil, Canada, China, India, Mexico, and before the European patent office. The presumptive term of the issued patents and pending patent applications is until 2030. Until one or more product candidates covered by a claim of one of these patents and patent applications are developed for commercialization, which may never occur, the company cannot evaluate the duration of any potential patent term extension under the Hatch-Waxman Amendments.
The company own issued patents in the United States, Mexico, New Zealand, South Africa and Russia claiming highly selective MC1r agonist peptides for treatment of inflammation-related diseases and disorders and related indications, and pending patent applications on two broad classes of highly selective MC1r agonist peptides in the United States, Australia, Brazil, Canada, China, India, Israel, Japan, Korea, and Mexico and before the European patent office. The presumptive term of the issued patents and pending patent applications is until 2030. Until one or more product candidates covered by a claim of one of these patent applications are developed for commercialization, which may never occur, the company cannot evaluate the duration of any potential patent term extension under the Hatch-Waxman Amendments.
The company own two issued United States patents claiming the PL-3994 substance and other natriuretic peptide receptor agonist compounds that Palatin has developed and an issued United States patent claiming a precursor molecule to the PL-3994 substance, both of which expire in 2027. Corresponding patents on the PL-3994 substance and other natriuretic peptide receptor agonist compounds were issued in Australia, Austria, Belgium, China, Colombia, Denmark, Finland, France, Germany, Hong Kong, Hungary, India, Ireland, Israel, Italy, Japan, Korea, Mexico, Netherlands, Philippines, Russia, South Africa, Spain, Sweden, Switzerland, and the United Kingdom, with terms until 2027. Patent applications on the PL-3994 substance and other natriuretic peptide receptor agonist compounds are pending in Brazil and Canada, with presumptive terms until 2027. Applications claiming precursor molecules for the PL-3994 substance and other compounds have issued in the United States, Australia, China, France, Germany, Hong Kong, India, Ireland, Israel, Japan, Mexico, Netherlands, Philippines, Korea, South Africa, Sweden, Switzerland and the United Kingdom, and expire in 2027. Patent applications on the precursor molecules are pending in Brazil, Canada, and before the Eurasian Patent Office, with presumptive terms until 2027. The company also own an issued United States patent claiming use of the PL-3994 substance for treatment of acute asthma and chronic obstructive pulmonary disease, which expires in 2031. The company do not know the full scope of patent coverage the company will obtain, or whether any patents will issue other than the patents already issued. Until one or more product candidates covered by a claim of the issued patents or one of these patent applications are developed for commercialization, which may never occur, the company cannot evaluate the duration of any potential patent term extension under the Hatch-Waxman Amendments.
The company additionally have 31 issued United States patents on melanocortin receptor specific peptides and small molecules, and five issued United States patents on natriuretic peptide receptor agonist compounds, but Palatin is not actively developing any product candidate covered by a claim of any of these patents.
In the event that a third party has also filed a patent application relating to an invention the company claimed in a patent application, the company may be required to participate in an interference proceeding adjudicated by the United States Patent and Trademark Office (“USPTO”) to determine priority of invention. The possibility of an interference proceeding could result in substantial uncertainties and cost, even if the eventual outcome is favorable to it. An adverse outcome could result in the loss of patent protection for the subject of the interference, subjecting it to significant liabilities to third parties, the need to obtain licenses from third parties at undetermined cost, or requiring it to cease using the technology.
Future Patent Infringement. The company do not know for certain that its commercial activities will not infringe upon patents or patent applications of third parties, some of which may not even have been issued. Although Palatin is not aware of any valid United States patents which are infringed by bremelanotide or PL-3994, the company cannot exclude the possibility that such patents might exist or arise in the future. The company may be unable to avoid infringement of any such patents and may have to seek a license, defend an infringement action, or challenge the validity of such patents in court. Patent litigation is costly and time consuming. If such patents are valid and the company do not obtain a license under any such patents, or Palatin is found liable for infringement, the company may be liable for significant monetary damages, may encounter significant delays in bringing products to market, or may be precluded from participating in the manufacture, use or sale of products or methods of treatment covered by such patents.
Proprietary Information. The company rely on proprietary information, such as trade secrets and know-how, which is not patented. Palatin has taken steps to protect its unpatented trade secrets and know-how, in part through the use of confidentiality and intellectual property agreements with its employees, consultants and certain contractors. If its employees, scientific consultants, collaborators or licensees develop inventions or processes independently that may be applicable to its product candidates, disputes may arise about the ownership of proprietary rights to those inventions and processes. Such inventions and processes will not necessarily become its property, but may remain the property of those persons or their employers. Protracted and costly litigation could be necessary to enforce and determine the scope of its proprietary rights.
If trade secrets are breached, its recourse will be solely against the person who caused the secrecy breach. This might not be an adequate remedy to it because third parties other than the person who causes the breach will be free to use the information without accountability to it. This is an inherent limitation of the law of trade secret protection.




