Trevena
Overview
Trevena, Inc. (TRVN) is a biopharmaceutical company developing innovative therapies based on breakthrough science to benefit patients and healthcare providers confronting serious medical conditions.
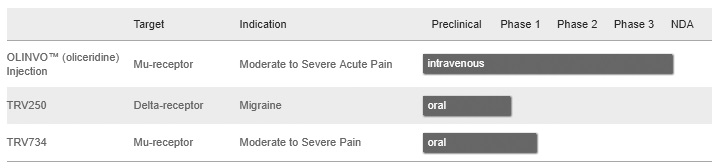
Using its proprietary product platform, Trevena has identified and are developing the following product candidates:1
- OLINVOTM (oliceridine) Injection: Trevena is developing OLINVO, a G protein biased ligand of the μ opioid receptor, for the management of moderate-to-severe acute pain where intravenous, or IV, administration is preferred. In February 2017, the company announced positive top-line results from its Phase 3 APOLLO-1 and APOLLO-2 pivotal efficacy studies of OLINVO in moderate-to-severe acute pain following bunionectomy and abdominoplasty, respectively. In both studies, all dose regimens achieved their primary endpoint of statistically greater analgesic efficacy than placebo, as measured by responder rate. In July 2017, the company announced that the company had completed enrollment in the Phase 3 open-label ATHENA safety study to support the new drug application, or NDA, for OLINVO. In the study, 768 patients were administered OLINVO to manage pain associated with a wide range of procedures and diagnoses. In January 2018, the company announced that the United States Food and Drug Administration, or FDA, had accepted the NDA the company submitted for OLINVO. The FDA also indicated that the Prescription Drug User Fee Act, or PDUFA, review date for the OLINVO NDA is November 2, 2018 and that it plans to hold an advisory committee meeting to discuss the NDA. If OLINVO ultimately receives regulatory approval, the company plan to commercialize it in the United States, either on its own or with a commercial partner, for use in acute care settings such as hospitals and ambulatory surgery centers; outside the United States, the company plan to commercialize OLINVO in certain countries with a commercial partner. The company currently hold all worldwide development and commercialization rights to OLINVO.
- TRV250: Trevena is developing TRV250, a G protein biased ligand targeting the δ-receptor, as a compound with a potential first-in-class, non-narcotic mechanism for the treatment of migraine. TRV250 also may have utility in a range of other central nervous system, or CNS, indications. Because TRV250 selectively targets the δ-receptor, the company believe it will not have the addiction liability of conventional opioids or other μ opioid related adverse effects like those seen with morphine or oxycodone. In the second quarter of 2017, the company began a Phase I study of TRV250 in the United Kingdom in healthy volunteers; the company expect to complete dosing in this study by the end of the first quarter of 2018.
Trevena has also identified and have completed the initial Phase 1 studies for TRV734, an orally administered new chemical entity expected to be used for first-line treatment of moderate-to-severe acute and chronic pain. The company intend to continue to focus its efforts for TRV734 on securing a development and commercialization partner for this asset.
Product Pipeline
OLINVO™ (oliceridine) Injection
OLINVO is a novel µ‑receptor G protein Pathway Selective modulator that activates the G protein pathway, which is associated with analgesia and avoids the β‑arrestin pathway, which is associated with limiting opioid analgesia and with
promoting opioid‑induced adverse events. Trevena is developing OLINVO for the management of moderate-to-severe acute pain where IV administration is preferred.
Disease and treatment options
The typical treatment paradigm in the U.S. for the management of moderate‑to‑severe acute pain is to initiate injectable or IV pain medication in the preoperative or immediate postoperative period to provide rapid and effective pain relief. Conventional IV opioid analgesics, such as morphine, fentanyl, and hydromorphone, are the mainstays of pain management in the immediate postoperative period and are approximately 50% of the injectable analgesic unit market. The effectiveness of conventional opioid agonists is limited because of severe side effects such as respiratory depression, nausea, vomiting, and constipation. Injectable non‑opioid analgesics are often used together with IV opioids for post‑surgical pain management; however, these drugs, such as IV non‑steroidal anti‑inflammatory drugs, or NSAIDs, IV acetaminophen, or local anesthetics such as bupivacaine, have potential cardiovascular, hepatic and gastrointestinal side effects. None of these non‑opioid analgesic approaches has displaced the use of opioid analgesics as the cornerstone of IV therapy for acute moderate-to-severe pain. The company believe that there remains significant unmet need for an effective analgesic agent with an improved safety and tolerability profile.
Clinical development
Trevena is developing OLINVO for the management of moderate‑to‑severe acute pain where IV administration is preferred. In the future, the company also may explore other formulations, such as transmucosal administration for breakthrough pain in additional, separate clinical trials. In the second quarter of 2017, the company held a successful Type B meeting with the FDA regarding the Chemistry, Manufacturing and Controls data package of its NDA submission for OLINVO. The company also held a successful pre-NDA meeting with the FDA regarding the clinical and non-clinical data package of the NDA in the second quarter of 2017.
Below is a summary of the clinical development work undertaken for OLINVO.
ATHENA Phase 3 Open Label Safety Study
The company conducted a Phase 3, open label, multicenter study evaluating the safety and tolerability of OLINVO in patients with moderate-to-severe pain caused by surgery or medical conditions. The trial was designed to model real-world use, including the use of multi-modal analgesia. Patients were treated with OLINVO on an as‑needed basis via IV bolus, patient‑controlled analgesia, or PCA, or both, as determined by the investigator. The primary objective was to assess the safety and tolerability of OLINVO. Pain intensity was measured as a secondary endpoint.
In the ATHENA study, 768 patients were treated with OLINVO. The most common procedures were orthopedic, gynecologic, colorectal, and general surgeries. Patients at elevated risk of opioid-related adverse events were well represented; more than 30% of patients were 65 years or older, and approximately 50% of patients were obese, with body mass index (BMI) >30 kg/m2. Only 2% of patients discontinued for adverse events, and 4% of patients discontinued for lack of efficacy. The most common adverse events were nausea, constipation, and vomiting, with prevalence lower than in the APOLLO studies. Adverse event rates associated with OLINVO administered by PCA and as-needed bolus dosing were similar, supporting the potential use of OLINVO in both administration paradigms.
APOLLO-1 and APOLLO-2 Phase 3 Studies
Trevena has conducted two pivotal efficacy trials evaluating OLINVO in patients with moderate-to-severe acute pain: the APOLLO-1 study, which evaluated pain for 48 hours following bunionectomy, and the APOLLO-2 study, which evaluated pain for 24 hours following abdominoplasty. In February 2017, the company announced positive top-line results from the APOLLO-1 and APOLLO-2 studies. In both studies, all dose regimens achieved the primary endpoint of statistically greater analgesic efficacy than placebo, as measured by responder rate.
The APOLLO-1 and APOLLO-2 studies were both Phase 3, multicenter, randomized, double-blind, placebo- and active-controlled studies of OLINVO. During the study period, a loading dose of placebo, morphine (4 mg), or OLINVO (1.5 mg) was administered first, and then patients used a PCA button to dose themselves as often as every 6 minutes with the same study drug: 1 mg morphine, or 0.1 mg, 0.35 mg, or 0.5 mg OLINVO. If PCA dosing was inadequate to control pain, patients could request supplemental study medication (2 mg morphine or 0.75 mg OLINVO, no more than once an hour). If the study medication regimen did not adequately manage pain, patients could opt for an NSAID rescue analgesic. Placebo loading, demand, and supplemental doses were volume-matched.
All endpoints were the same in both studies, except that dosing and pain assessment were for 48 hours in APOLLO-1 and 24 hours in APOLLO-2. Efficacy was measured by a responder analysis, which defined a responder as a patient who experienced at least a 30% reduction in their sum of pain intensity difference at the end of the treatment period without either early discontinuation (for lack of efficacy or safety/tolerability) or use of rescue medication. Non-inferior efficacy compared to morphine and superior efficacy compared to morphine were key secondary endpoints. Respiratory safety events were defined as clinically relevant worsening of respiratory status, including oxygen saturation, respiratory rate, or sedation. The product of the frequency and conditional duration of these events was reported as respiratory safety burden, a key secondary endpoint. Additional measures of respiratory safety included prevalence of oxygen saturation less than 90% and prevalence of supplemental oxygen use. Measures of gastrointestinal tolerability included use of rescue antiemetics, vomiting, and spontaneously reported nausea.
APOLLO-1 (bunionectomy)
- All three OLINVO regimens (0.1 mg, 0.35 mg, and 0.5 mg on-demand doses) achieved the primary endpoint with statistically superior responder rates compared to placebo at 48 hours (p<0.0001, adjusted for multiplicity).
- The 0.35 mg and 0.5 mg OLINVO dose regimens demonstrated efficacy comparable to morphine at 48 hours based on responder rate (both doses p<0.005 for non-inferiority to morphine). Both doses were also comparable to morphine for rates of rescue analgesic use.
- Following the 1.5 mg initial loading dose, all OLINVO regimens demonstrated rapid onset with statistically significant efficacy within 5 minutes (p<0.05).
- OLINVO exhibited a dose-related trend of improved respiratory safety burden in all three OLINVO dose regimens (p<0.05 for the 0.1 mg regimen vs. morphine). Consistent with this, in all dose regimens OLINVO showed dose-related trends of reduced respiratory safety events (P<0.05 for 0.1 mg and 0.35 mg regimens vs. morphine), prevalence of oxygen desaturation (O2<90%) and lower prevalence of supplemental oxygen use (p<0.05 for the 0.1 mg regimen vs. morphine for both measures).
- OLINVO exhibited less antiemetic use compared to morphine (p<0.05 for all OLINVO regimens vs. morphine). Consistent with this, OLINVO showed dose related trends of lower prevalence of nausea and vomiting in all three OLINVO regimens (p<0.05 for the 0.1 mg regimen vs. morphine).
APOLLO-2 (abdominoplasty)
- All three OLINVO dose regimens achieved the primary endpoint with statistically superior responder rates compared to placebo at 24 hours (adjusted p<0.05 for the 0.1 mg regimen; adjusted p<0.001 for the 0.35 mg and 0.5 mg regimens).
- he 0.35 mg and 0.5 mg OLINVO dose regimens demonstrated efficacy comparable to morphine at 24 hours based on responder rate (p<0.05 for non-inferiority of the 0.35 mg regimen vs. morphine). Both doses were also comparable to morphine for rates of rescue analgesic use.
- Following the 1.5 mg initial loading dose, all OLINVO regimens demonstrated rapid onset with statistically significant efficacy within 5 to 15 minutes (p<0.05).
- OLINVO showed a dose-related trend of improved respiratory safety burden in all three OLINVO dose regimens (p<0.05 for the 0.1 mg regimen vs. morphine). Consistent with this, for all dose regimens OLINVO showed dose-related trends of reduced respiratory safety events, prevalence of oxygen desaturation (O2<90%) and lower prevalence of supplemental oxygen use (p<0.05 for the 0.1 mg regimen vs. morphine for all measures).
- OLINVO showed a dose-related trend of less antiemetic use than morphine for all three OLINVO regimens (p<0.05 for the 0.1 mg OLINVO regimen vs. morphine). Consistent with this, OLINVO showed dose-related trends of lower prevalence of nausea and vomiting (p<0.05 for the 0.1 mg regimen vs. morphine for both nausea and vomiting; p<0.05 for the 0.35 mg regimen vs. morphine for vomiting).
In both studies, OLINVO was generally well-tolerated. The most common drug-related adverse events, or AEs, were nausea, vomiting, headache, and dizziness.
Phase 2b trial of OLINVO in acute postoperative pain following abdominoplasty
The aim of its Phase 2b clinical trial was to evaluate the efficacy, safety and tolerability of OLINVO in the management of postoperative pain using morphine as a benchmark, utilizing on‑demand dosing to reflect standard clinical practice. This Phase 2b trial was a randomized, double‑blind, placebo‑ and active‑controlled trial of OLINVO in which the company enrolled 200 patients with moderate-to-severe acute postoperative pain after abdominoplasty surgery. Two regimens of OLINVO were tested: the first consisted of a 1.5 mg intravenous loading dose with 0.1 mg self‑administered on‑demand doses as often as every six minutes using a PCA device; the second consisted of a 1.5 mg loading dose with 0.35 mg on‑demand doses as often as every six minutes using a PCA device. A commonly used morphine PCA regimen also was tested, consisting of a 4 mg loading dose with 1 mg on‑demand doses as often as every six minutes. Placebo was administered as a loading dose and on‑demand doses were volume‑matched to the active regimens. Rescue medication consisting of ibuprofen or oxycodone was used in all groups.
In August 2015, the company reported top‑line results from this trial. OLINVO demonstrated statistically significant pain reduction compared to placebo and comparable efficacy to morphine. OLINVO provided rapid reduction in average pain scores, consistent with the previous Phase 2 trial where OLINVO showed more rapid onset of meaningful pain relief than morphine. Rescue analgesic use was similar for both OLINVO and morphine, and less than half the rate of rescue analgesic use for placebo. In this study, the OLINVO groups had a significantly lower prevalence (percentage of patients) of hypoventilation events (a measure of respiratory safety), nausea, and vomiting than the morphine group. The most frequently reported AEs, associated with OLINVO were nausea, vomiting, hypoventilation and headache. Opioid‑related AEs were generally less frequent in the OLINVO groups compared to morphine. No drug‑related serious adverse events were reported in the study.
Phase 2a/b trial of OLINVO in acute postoperative pain following bunionectomy
The aim of its Phase 2a/b clinical trial was to evaluate the efficacy and tolerability of OLINVO in the management of postoperative pain using morphine as a benchmark, using fixed dose and dose interval to characterize the performance of OLINVO. The trial was a multicenter, randomized, double‑blind, placebo‑ and active‑controlled, multiple dose, adaptive trial in 333 women and men undergoing a primary unilateral first‑metatarsal bunionectomy surgery at four sites in the United States. Patients were randomized after surgery to receive OLINVO, morphine or placebo to manage their pain. Pain intensity was measured using validated numeric rating scales ranging from ten (most severe pain) to zero (no pain) at multiple time points up to 48 hours. Based on these scales, analgesic efficacy was assessed with a time‑weighted average change in pain score over 48 hours—a well‑established measure of changes in the intensity of pain over time and an FDA‑recommended endpoint for pain studies.
In November 2014, the company announced top‑line data from this trial. At doses of 2 mg and 3 mg of OLINVO administered every three hours, the trial achieved its primary endpoint of statistically greater pain reduction than placebo for 48 hours, which the company believe demonstrated proof of concept for OLINVO. Over the 48‑hour trial period, the 3 mg dose of OLINVO administered every three hours also showed statistically superior analgesic efficacy compared to the 4 mg dose of morphine administered every four hours. Additionally, in the first three hours of dosing, when pain was most severe, the 1 mg, 2 mg, and 3 mg doses of OLINVO demonstrated superior analgesic efficacy in the trial compared to placebo, and the 2 mg, and 3 mg doses of OLINVO demonstrated superior analgesic efficacy compared to the 4 mg dose of morphine.
There were no serious AEs reported in the trial. Both the 2 mg and 3 mg doses of OLINVO showed overall tolerability over the 48‑hour trial period similar to that of the 4 mg dose of morphine administered every four hours. The most frequently reported adverse events associated with OLINVO were dizziness, headache, somnolence, nausea, vomiting, flushing and itching. Adverse effects were generally dose‑related.
Phase 1 clinical studies of OLINVO
The company also have completed a number of Phase 1 clinical studies of OLINVO. These included two single ascending dose studies of OLINVO given as a 60 minute continuous infusion or a 2 minute bolus infusion that showed dose‑related increases in plasma exposure and pupil constriction, a biomarker for CNS opioid activity across a range of doses that were generally well tolerated. Because in vitro data suggest that OLINVO is metabolized by at least two liver enzymes, CYP2D6 and CYP3A4, the company assessed OLINVO pharmacokinetics, pharmacodynamics, safety and tolerability in CYP2D6 “poor metabolizer” healthy volunteers with little to no CYP2D6 activity. This study showed that OLINVO clearance was reduced by approximately 50% in the poor metabolizers suggesting that a lower frequency of dosing may be required to offer effective pain relief.
In 2013, the company completed a Phase 1b proof of concept exploratory trial in healthy male subjects. The aims of this trial were to characterize the analgesic efficacy and safety and tolerability of a single dose of OLINVO as compared to a single 10 mg dose of morphine. The company used a well‑established evoked‑pain model, the cold pain test, to evaluate the analgesic effects of OLINVO by measuring the time to hand removal, or latency, from a temperature‑controlled cold water bath. At both the 3.0 mg and 4.5 mg doses, OLINVO showed superior efficacy as compared to a 10 mg morphine dose that was statistically significant with a p‑value of less than 0.05 at the ten and 30 minute time points after dosing. The durability of the analgesic effect was similar to morphine. In addition, the time to peak effect was more rapid than that for morphine. Overall, OLINVO was well tolerated in the trial. Subjects receiving OLINVO showed less severe nausea and less frequent vomiting at the 1.5 mg and 3.0 mg doses as compared to a 10 mg dose of morphine. OLINVO also showed less respiratory depression compared to morphine over 4 hours.
In October 2014 the company completed an adaptive, multiple ascending dose study of OLINVO in more than 50 healthy subjects. The safety, tolerability, pharmacokinetic and pharmacodynamics results of this study were consistent with the early Phase 1 studies described above. Recently, the company also successfully completed an absorption, distribution, metabolism, and excretion study, with results consistent with the lack of known active OLINVO metabolites. The company also completed a QTc interval study, which showed no evidence of concentration-related QTc changes, a renal impairment study which showed no evidence of altered PK/accumulation in patients with renal failure compared to healthy patients, and a human abuse liability study which showed that OLINVO had similar abuse liability as IV morphine when administered at a comparably analgesic dose.
Regulatory
In December 2015, the FDA granted Fast Track designation to OLINVO for the management of moderate‑to‑severe acute pain. The Fast Track program is designed to facilitate the development and review of drugs intended to treat serious conditions with unmet medical needs by providing sponsors with the opportunity for frequent interactions with the FDA. In February 2016, the FDA granted Breakthrough Therapy designation to OLINVO for the management of moderate‑to‑severe acute pain. Breakthrough Therapy designation is granted by the FDA to new therapies intended to treat serious conditions and for which preliminary clinical evidence indicates that the drug may demonstrate substantial clinical improvement over available therapies. Breakthrough Therapy designation provides all the benefits of the Fast Track program, as well as more intensive FDA guidance on preparing an efficient drug development program. In January 2018, the company announced that the FDA had accepted for review the NDA the company submitted for OLINVO. The FDA also indicated that the PDUFA review date for the OLINVO NDA is November 2, 2018 and that it plans to hold an advisory committee meeting to discuss the NDA.
Commercialization
According to 2015 IMS data, approximately 51 million patients in the United States were treated with an IV opioid in the hospital setting. The majority of use is in the inpatient setting where approximately 16 million patients are treated for an average of two days. The World Health Organization estimates that over 230 million major surgical procedures are performed each year worldwide. The Centers for Disease Control and Prevention, or CDC, estimate that 100 million surgical and invasive diagnostic procedures occur annually in the United States. Accordingly, if approved, the company believe that there is a large potential commercial opportunity for OLINVO in the management of both surgical and medical acute pain.
If OLINVO ultimately receives regulatory approval, the company plan to commercialize it in the United States, either on its own or with a commercial partner. Assuming approval on the November 2, 2018 PDUFA review date and allowing for DEA scheduling of OLINVO within 90 days of FDA approval (as mandated by the Improving Regulatory Transparency for New Medicinal Therapies Act), the company anticipate launching OLINVO in the first quarter of 2019. Outside the United States, the company expect to seek collaborators to commercialize OLINVO to offset risk and preserve capital.
To commercialize OLINVO in the United States, the company intend to utilize a hospital-focused specialty sales force targeting surgeons, anesthesiologists, hospitalists, and other healthcare providers with acute post-surgical or medical pain management responsibility. The company believe the greatest opportunity for OLINVO will be in the hospital inpatient setting where the company estimate that approximately 50% of the 16 million patients may be at higher risk of opioid-related adverse events such as respiratory depression or post-operative nausea and vomiting. These events are common and result in a significant cost burden to the hospital system. The company believe that many of these patients are complex, and have comorbid conditions. Population and hospital trend data indicates that these patient groups will continue to grow and be an area of focus for inpatient care. The company expect that the aging population will also continue to drive surgical volumes and hospital length of stay will increase. Elderly patients are also twice at risk of experiencing an opioid-related adverse event.
Approximately 1,200 US hospitals are responsible for 70% of the annual volume of conventional IV opioid drugs prescribed. Trevena has selected a subset of these hospitals that have rapidly adopted new branded agents in the past as the initial account targets for OLINVO at launch. The company will work to secure Pharmacy and Therapeutics Committee approval and subsequent utilization of OLINVO at these hospitals. Because many of its priority customers also provide care in other hospital settings, the company anticipate that the company will target a select number of hospital outpatient departments and ambulatory surgery centers. Given the changing dynamics in the hospital marketplace and the increased emphasis on clinical and economic outcomes, the company expect its commercialization plans to include health economic information designed to demonstrate the value of OLINVO through a potential reduction in adverse events related to the use of conventional IV opioids.
Manufacturing
Trevena has completed process development of the active pharmaceutical ingredient, or API, and have manufactured multiple commercial scale batches using its proposed commercial process under current good manufacturing practices, or cGMP, conditions. The company also have completed drug product process development and have manufactured multiple batches of drug product using the proposed commercial process under cGMP conditions.
For OLINVO, Trevena has established commercial supply agreements for the manufacture of the API and finished (compounded, filled and packaged) drug product. Alcami Corporation, or Alcami, is contracted to supply 100% of its commercial API from its Germantown, WI manufacturing facility. Trevena has existing commercial supply agreements with two separate companies for the supply of drug product. Alcami is contracted to supply commercial drug product from its facilities in Charleston, SC and Wilmington, NC. Pfizer CentreOne (formerly Hospira) is also contracted to supply commercial drug product from its facility in McPherson, KS. The company anticipate that OLINVO will be classified as a Schedule II controlled substance. All third-party facilities throughout the supply chain have the appropriate DEA licenses for handling Schedule II controlled substances according to each of their respective contractual roles (manufacturing, testing, distribution, etc.).
Competition
If OLINVO is approved for IV management of moderate-to-severe acute pain, it will compete with generic IV opioid analgesics, such as morphine, hydromorphone and fentanyl. The analgesic effectiveness of these agents is limited by well‑known adverse side effects, such as respiratory depression, nausea, vomiting, constipation, and post‑operative ileus. OLINVO also may compete against, or be used in combination with, OFIRMEV® (IV acetaminophen), marketed by Mallinckrodt plc, EXPAREL® (liposomal bupivacaine), marketed by Pacira Pharmaceuticals, Inc., CALDOLOR® (IV ibuprofen), marketed by Cumberland Pharmaceuticals, and DYLOJECT™ (IV diclofenac) marketed by Hospira. Together with generic versions of IV NSAIDs such as ketorolac, and generic versions of local anesthetics such as bupivacaine, these non‑opioid analgesics are currently used in combination with opioids in the multimodal management of moderate-to-severe acute pain.
The company also are aware of a number of products in mid- and late-stage clinical development that are aimed at improving the treatment of moderate-to-severe acute pain and may compete with OLINVO. AcelRx Pharmaceuticals, Inc. is developing a range of acute pain products involving sufentanil oral nanotabs in hand‑held dispensers including DSUVIA™ and ZALVISO™. Innocoll Holdings plc. and Heron Therapeutics Inc. have proprietary long‑acting reformulations of bupivacaine in development. Recro Pharma, Inc. is developing an IV version of the NSAID meloxicam. Cara Therapeutics Inc. is developing IV and oral dose forms of a peripherally restricted Κ‑opioid receptor agonist, which has been administered in combination with µ‑opioids in clinical trials. Avenue Therapeutics, Inc. is developing an IV version of generic opioid tramadol for moderate-to-severe acute pain.
Intellectual property
The company's OLINVO patent portfolio is wholly owned by it. The portfolio includes three issued U.S. patents (U.S. Patent Nos. 8,835,488, 9,309,234, and 9,642,842), which claim, among other things, OLINVO, compositions comprising OLINVO, and methods of using OLINVO. The issued patents are expected to expire no earlier than 2032, subject to any disclaimers or extensions, and any U.S. patent to issue in the future is also expected to expire no earlier than 2032, subject to any disclaimers or extensions. The company also have issued patents in Australia, China, Europe, Hong Kong, Eurasia, Japan, and New Zealand, which claim among other things, OLINVO, compositions comprising OLINVO and methods of making or using OLINVO. The foreign portfolio also includes an application that has been allowed by the European Patent Office, which claim among other things, OLINVO, compositions comprising OLINVO and methods of using OLINVO. Trevena has patent applications pending in the United States, South Korea, Brazil, Canada, Israel, India, and Hong Kong. The issued patents and patents that could issue in the future from these allowed or pending applications outside the United States are expected to expire no earlier than 2032, subject to any disclaimers or extensions.
TRV250
TRV250 is under investigation as a potential new mechanism of action for the treatment of acute migraine. The first-time-in-human Phase 1 trial was a single ascending dose study of safety, tolerability, and pharmacokinetics of subcutaneous TRV250 in healthy volunteers, and included a separate cohort who received a single oral dose of TRV250. In November, the Company announced positive interim results for the initially planned doses, which demonstrated dose-proportional exposure after subcutaneous administration and adequate oral bioavailability to support further clinical development. Following this interim readout, the Company extended the trial to study additional subcutaneous doses. Dosing is now complete, having reached a top subcutaneous dose of 30 mg. The Company expects to release data in the coming months.
Based on the profile of TRV250, the company believe it has the potential to be a first‑in‑class treatment for migraine. According to Decision Resources, a healthcare consulting company, the acute episodic migraine market encompassed approximately 12 million drug‑treated patients in 2013 in the United States, representing approximately $2.2 billion of sales. The company estimate that approximately 20% to 30% of these patients either do not respond to, or cannot tolerate, the market‑leading triptan drug class, and an additional 30% would benefit from improved efficacy compared to these drugs.
Triptans, a generic family of 5HT1B agonists, are the current standard treatment for acute treatment of migraine, and account for 80% of migraine therapies prescribed during physician office visits. Other less commonly prescribed acute treatments include ergot alkaloids, and analgesics such as opioids and NSAIDs. Various branded reformulations of triptan molecules have been launched, and Trevena is aware of others in development. In May 2016, Avanir Pharmaceuticals, Inc. launched a dry powder nasal delivery formulation of sumatriptan, called ONZETRA™ Xsail™. RedHill Biopharma, Ltd. and IntelGenx Corp. resubmitted the 505(b)(2) NDA for RIZAPORT®, an oral thin film rizatriptan formulation, to the FDA in November 2017. In addition, Allergan is developing an orally inhaled formulation of dihydroergotamine, called Semprana™. Lasmiditan, a selective 5HT1F agonist, is in late stage development by Colucid Pharmaceuticals, Inc., recently acquired by Eli Lilly and Company. Allergan (ubrogepant) and Biohaven (rimegepant) both have an oral anti-calcitonin gene-related peptide, or CGRP, antagonist in Phase 3 testing for the acute treatment of migraine.
Patients suffering from frequent or chronic migraine headaches may also use preventative agents to decrease the frequency and severity of migraines. Botox® is currently the most widely prescribed migraine prophylactic, but certain anticonvulsants, such as topiramate, and beta-blockers, such as propranolol, are also used. Trevena is aware of four companies with anti-CGRP antibody products in late stage development for preventative treatment of migraine: Amgen, Inc. has submitted a biologics license application, or BLA, to the FDA for Aimovig (erenumab), which will be co-commercialized with Novartis; Eli Lilly and Company has submitted a BLA for galcanezumab; Teva Pharmaceutical Industries Limited has submitted a BLA for fremanezumab; and Alder BioPharmaceuticals Inc. is completing Phase 3 trials with eptinezumab. Allergan also has an oral CGRP antagonist molecule, atogepant, in Phase 2 trials for migraine prevention.
The company believe its preclinical data support targeting the δ-receptor for the treatment of CNS disorders. Prior approaches to modulate this receptor have been limited by a significant risk of seizure associated with this target. By contrast, TRV250 is a potent δ-receptor ligand that displayed strong efficacy in animal models of migraine and other CNS disorders with reduced seizure liability through selectively activating G protein coupling without engaging β‑arrestin. These in vivo data are further supported by data for δ‑agonists in β‑arrestin knockout mice suggesting that β‑arrestin plays a role in seizures. In the future, the company may decide to seek a collaborator for TRV250 with CNS development and commercialization expertise outside the United States.
Trevena has one non-provisional patent application in the United States directed to compounds that modulate the δ-receptor. This application is solely owned by it. Trevena has also filed, or are in the process of filing, foreign national phase applications in Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, and New Zealand. Any patents that may issue from these applications are expected to expire no earlier than 2036, subject to any disclaimers or extensions.
TRV734
TRV734 is a small molecule G protein biased ligand of the µ opioid receptor that the company discovered and have developed through Phase 1 as a first‑line, orally administered compound for the treatment of moderate-to-severe acute and chronic pain. Like OLINVO, TRV734 takes advantage of a well‑established mechanism of pain relief by targeting the µ opioid receptor, but does so with enhanced selectivity for the G protein signaling pathway, which in preclinical studies was linked to analgesia, as opposed to the β‑arrestin signaling pathway, which in preclinical studies was associated with side effects. Subject to successful preclinical and clinical development and regulatory approval, the company believe TRV734 may have an improved efficacy and side effect profile as compared to current commonly prescribed oral analgesics, such as oxycodone. The company intend to continue to focus its efforts for TRV734 on securing a worldwide development and commercialization partner for this asset.
TRV734 has shown a similar profile to OLINVO in in vitro and in vivo studies. It is highly selective for the µ opioid receptor where, like the most powerful opioid analgesics, it is a strong agonist of G protein coupling. TRV734 is distinct from those analgesics in its very weak recruitment of β‑arrestins to the µ opioid receptor. In its preclinical studies, TRV734 showed analgesic effects in preclinical pain models similar to oxycodone and morphine. In the same studies, TRV734 caused less constipation compared to equivalently analgesic doses of oxycodone and morphine. TRV734 is active after oral administration in mice and rats, has high oral bioavailability and has been well tolerated in non‑human primates. Trevena has completed three Phase 1 trials of TRV734 in healthy volunteers, including a single ascending dose study, a multiple ascending dose study, and a pharmacokinetic study. In these studies, a total of 127 healthy volunteers were exposed to TRV734 at doses between 2 mg and 250 mg. The company incorporated measures to assess the potential for analgesic efficacy and tolerability advantages in these studies. Based on these data and data for OLINVO, the company believe that TRV734 may offer an improved efficacy profile as compared to current opioid therapies or equivalent efficacy with an improved gastrointestinal tolerability and respiratory safety profile.
The company's TRV734 patent portfolio is wholly owned by it and includes one issued U.S. patent (U.S. Patent No, 9,044,469) claiming TRV734, other compounds and/or methods of making or using the same. This patent is expected to expire no earlier than 2032, subject to any disclaimers or extensions. The company also have issued patents in Australia, China, Europe, Eurasia, Hong Kong, Israel, Japan, and New Zealand claiming TRV734, other compounds and/or methods of making or using the same. The company also have patent applications pending in South Korea, Brazil, Canada, Israel, India, and Hong Kong. The issued patents and patents that could issue in the future from these allowed or pending applications outside the United States are expected to expire no earlier than 2032, subject to any disclaimers or extensions.
TRV027
TRV027 is a peptide β‑arrestin biased ligand that targets the angiotensin II type 1 receptor (AT1R), inhibiting angiotensin II‑mediated G protein signaling and activating β‑arrestin signaling. On May 3, 2013, the company entered into an option agreement and a license agreement with Allergan plc (formerly Actavis plc and Forest Laboratories Holdings Limited), under which the company granted to Allergan an exclusive option to license TRV027. In March 2015, the company signed a letter agreement with Allergan pursuant to which Allergan paid it $10.0 million to fund the expansion of the Phase 2b (BLAST-AHF) trial of TRV027 in AHF from 500 patients to 620 patients. In May 2016, the company announced that TRV027 did not meet either the primary or secondary endpoints of this Phase 2b (BLAST-AHF) trial. In August 2016, Allergan notified it of its decision to not exercise its exclusive option. As such, Trevena has retained all rights to TRV027. At this time, Trevena has no plans for further clinical development of TRV027.
The company's TRV027 patent portfolio is wholly owned by it. The portfolio includes four issued U.S. patents (U.S. Patent Nos. 8,486,885; 8,796,204; 8,809,260; and 8,993,511) that claim, among other things, TRV027, compositions comprising TRV027, and methods of using TRV027. The company also have issued patents in Europe, Australia, Japan, New Zealand, China, and Hong Kong. The issued U.S. patents covering the composition of matter and methods of using TRV027 are expected to expire no earlier than 2031 (U.S. Patent No. 8,486,885) and 2029 (U.S. Patent Nos. 8,796,204; 8,809,260; and 8,993,511), subject to any disclaimers or extensions. The issued European Patent is expected to provide coverage for TRV027 throughout most of European Union until at least 2029, subject to any disclaimers or extensions.
S1P Modulators
In July 2017, the company disclosed a new preclinical lead optimization program targeting S1P receptors. The company's compounds are all new chemical entities are, expected to be non-addictive, and use a new mechanism of action that in preclinical models avoids the immune suppression associated with approved and investigational S1P receptor targeted drugs. These molecules have demonstrated activity in preclinical models of chemotherapy-induced peripheral neuropathy, neuropathic pain, and inflammatory pain. The company expect to complete characterization of the lead compounds in 2018 to determine if any merit IND-enabling studies to support Phase 1 clinical trials.
Trevena is aware of a certain U.S. patent owned by a third party with claims that are broadly directed to a method of treating chemotherapy induced neuropathic pain with an S1P receptor agonist or an S1P receptor antagonist. Although the company do not believe that this is a valid patent, this patent could be construed to cover its S1P compounds.
Intellectual Property
The company strive to protect the proprietary technologies that the company believe are important to its business, including seeking and maintaining patent protection intended to cover the composition of matter of its product candidates, their methods of use, related technology and other inventions that are important to its business. The company also rely on trade secrets and careful monitoring of its proprietary information to protect aspects of its business that are not amenable to, or that the company do not consider appropriate for, patent protection.
The company's success will depend significantly on its ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know how related to its business, defend and enforce its patents, maintain its licenses to use intellectual property owned by third parties, preserve the confidentiality of its trade secrets and operate without infringing valid and enforceable patents and other proprietary rights of third parties. The company also rely on know how, and continuing technological innovation to develop, strengthen and maintain its proprietary position in the field of modulating GCPRs with biased ligands.
One or more third parties may hold intellectual property, including patent rights, that is important or necessary to the development of its products. It may be necessary for it to use the patented or proprietary technology of third parties to commercialize its products, in which case the company would be required to obtain a license from these third parties on commercially reasonable terms, or its business could be harmed, possibly materially. If the company were not able to obtain a license, or were not able to obtain a license on commercially reasonable terms, its business could be harmed, possibly materially.
The company plan to continue to expand its intellectual property estate by filing patent applications directed to dosage forms, methods of treatment for its product candidates. The company anticipate seeking patent protection in the United States and internationally for compositions of matter covering the compounds, the chemistries and processes for manufacturing these compounds and the use of these compounds in a variety of therapies.
The patent positions of biopharmaceutical companies like it are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and the patent’s scope can be modified after issuance. Consequently, the company do not know whether any of its product candidates will be protectable or remain protected by enforceable patents. The company cannot predict whether the patent applications Trevena is currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that the company hold may be challenged, circumvented or invalidated by third parties.
Because many patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, the company cannot be certain that the company will be able to obtain patent protection for the inventions disclosed and/or claimed in its pending patent applications. Moreover, the company may have to participate in interference proceedings declared by the United States Patent and Trademark Office or a foreign patent office to determine priority of invention or in post grant challenge proceedings, such as oppositions, inter partes review, post grant review or a derivation proceeding, that challenge its entitlement to an invention or the patentability of one or more claims in its patent applications or issued patents. Such proceedings could result in substantial cost, even if the eventual outcome is favorable to it.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which the company file, the patent term is 20 years from the earliest date of filing a PCT application or a non provisional patent application, subject to any disclaimers or extensions. The term of a patent in the United States can be adjusted and extended due to the failure of the United States Patent and Trademark Office following certain statutory and regulation deadlines for issuing a patent.
In the United States, the patent term of a patent that covers an FDA approved drug also may be eligible for patent term extension, which permits patent term restoration as compensation for a portion of the patent term lost during clinical development and the FDA regulatory review process. The Hatch Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under clinical development and regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other non United States jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when its pharmaceutical products receive FDA approval, the company expect to apply for patent term extensions on patents covering those products. Although, the company intend to seek patent term extensions to any of its issued patents in any jurisdiction where these are available there is no guarantee that the applicable authorities, including the United States Patent and Trademark Office, will agree with its assessment of whether such extensions should be granted, and even if granted, the length of such extensions.
The company also rely on trade secret protection for its confidential and proprietary information. Although the company take steps to protect its proprietary information and trade secrets, including through contractual means with its employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to its trade secrets or disclose its technology. Thus, the company may not be able to meaningfully protect its trade secrets. It is its policy to require its employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with it. These agreements provide that all confidential information concerning its business or financial affairs developed or made known to the individual during the course of the individual’s relationship with it is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual, and which are related to its current or planned business or research and development or made during normal working hours, on its premises or using its equipment or proprietary information, are its exclusive property.
Manufacturing
The company do not own or operate any manufacturing facilities. The company currently rely, and expect to continue to rely, on third parties for the manufacture of its product candidates for preclinical and clinical testing, as well as for commercial manufacture if its product candidates receive marketing approval.
Commercialization
Trevena has not yet fully established sales, marketing or product distribution infrastructure. Subject to successfully completing product development and receiving marketing approvals, the company expect to commence commercialization activities for its wholly owned products by insourcing or outsourcing a sales organization, initially in the hospital market, or by seeking a commercial partner in the United States. If the company choose to insource or outsource a sales organization, the company believe that it will be able to address the community of physicians who are the key specialists in treating the patient populations for which its product candidates are being developed. Outside the United States, the company expect to enter into distribution and other commercial arrangements with third parties for any of its product candidates that obtain marketing approval. The company also intend to license out commercial rights for products that require a substantial primary care presence. In parallel with building its commercial organization, the company plan to develop educational initiatives with respect to approved products and relationships with thought leaders in relevant fields of medicine.




