Vaxart Inc
Summary
- Vaxart is a clinical-stage biotechnology company developing a range of oral recombinant vaccines based on its proprietary delivery platform.
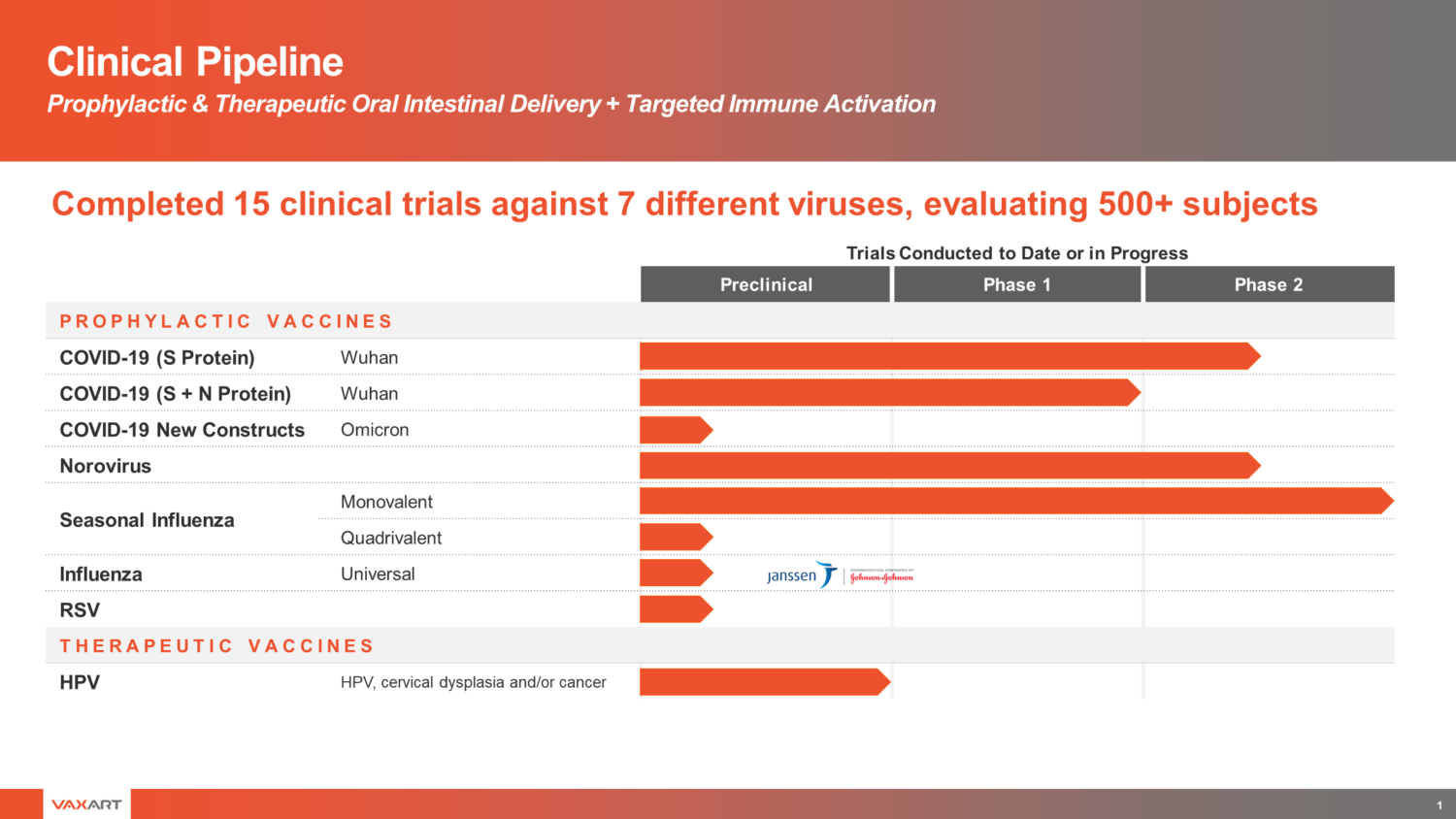
- Its development programs currently include pill vaccines designed to protect against coronavirus, norovirus, seasonal influenza and respiratory syncytial virus (RSV), as well as a therapeutic vaccine for human papillomavirus (HPV), Vaxart’s first immuno-oncology indication.
- Vaxart vaccines are designed to be administered using tablets that can be stored and shipped without refrigeration and eliminate the risk of needle-stick injury.

Vaxart (NASDAQ: VXRT) is a clinical-stage biotechnology company developing a range of oral recombinant vaccines based on its proprietary delivery platform.
Its development programs currently include pill vaccines designed to protect against coronavirus, norovirus, seasonal influenza and respiratory syncytial virus (RSV), as well as a therapeutic vaccine for human papillomavirus (HPV), Vaxart’s first immuno-oncology indication.
Financial Highlights
Third Quarter 2022 Financial Results
November 08, 2022; Vaxart, Inc. issued its business update today for the third quarter of 2022.1
Vaxart ended the third quarter with cash, cash equivalents and available-for-sale debt securities of $114.8 million, compared to $131.5 million as of June 30, 2022. The decrease was primarily due to $14.6 million of cash used in operations.
The Company reported a net loss of $29.3 million for the third quarter of 2022, compared to $17.6 million for the third quarter of 2021. Net loss per share for the third quarter of 2022 was $0.23, compared to a net loss of $0.14 per share in the third quarter of 2021. The increase in net loss was primarily due to a significant increase in research and development costs.
Research and development expenses were $22.5 million for the third quarter of 2022, compared to $12.4 million for the third quarter of 2021. The increase was mainly due to increases in headcount and related costs, and in manufacturing and clinical trial expenses related to its COVID-19 and norovirus vaccine candidates.
General and administrative expenses were $7.0 million for the third quarter of 2022, compared to $5.0 million for the third quarter of 2021. The increase was mainly due to increases in headcount and related costs and in legal and professional costs.
COVID-19 Vaccine Developments
In September 2022, Vaxart reported positive top-line data from its Phase II COVID-19 trial supporting broad potential of the Company’s COVID-19 vaccine candidates to tackle the challenges of an evolving virus that continues to overcome the immune protection provided by approved vaccines.
Vaxart is the only company with a mucosal vaccine candidate for COVID-19 that has produced Phase II clinical data that shows it stimulates mucosal immunity.
In July 2022, the Company updated Phase I data showing Vaxart’s Spike/Nucleocapsid (S+N) candidate stimulated SARS-CoV-2-specific IgA antibodies in saliva and nasal samples from human subjects and was cross-reactive to many different coronaviruses that are more divergent than circulating variants of SARS-CoV-2.
Norovirus Vaccine Developments
In June 2022, Vaxart reported positive top-line data about its norovirus vaccine candidate.
The data from Vaxart’s Phase Ib trial in subjects aged 55-80 demonstrated that Vaxart’s oral norovirus vaccine candidate stimulated a robust immune response across all doses, with a dose-dependent production of IgA antibody secreting cells.
Results were consistent with previous studies conducted in younger populations, which is typically not the case, as the immune system often weakens with age, and older people tend to have less robust responses to vaccination than younger people.
No vaccine exists in the United States to treat norovirus, a virus that causes up to 21 million cases, 109,000 hospitalizations and 900 deaths annually in the United States.
Corporate Updates
Bolstered management and Board with three significant additions:
- In August, named Ray Stapleton, Ph.D. as Chief Technology Officer.
- Dr. Stapleton joins Vaxart from Genocea, where he served as CTO and Executive Vice President, working to develop next generation personalized immunotherapies in the forms of vaccines and cell therapies. His prior experience includes senior manufacturing and technical operations roles at a number of biotech companies after spending 15 years in positions of increasing responsibility in Merck and Company’s manufacturing organization.
- Also in August, appointed Elaine J. Heron, Ph.D. and W. Mark Watson to the Company’s Board of Directors.
- Dr. Heron currently serves on the boards of BioMarin Pharmaceutical, Inc., Palvella Therapeutics, Inc., Visgenx, Inc., and Watershed Medical, Inc. She also serves as an advisor to Kyto Technology and Life Science, Inc. Dr. Heron has over 35 years of experience in the life science research and biotech development sectors.
- Mr. Watson is a Certified Public Accountant with more than 40 years of experience in public accounting and auditing, having spent his entire career from January 1973 to June 2013 at Deloitte Touche Tohmatsu and its predecessor, most recently as Central Florida Marketplace Leader.
Planned Clinical Milestones in the COVID-19 and Norovirus Pipelines
Vaxart continues to make progress on its expected milestones:
- Start of Phase II trial of Vaxart’s bivalent norovirus vaccine candidate in Q4 2022 or Q1 2023.
- Top-line data from ongoing Phase II norovirus challenge study expected at the end of Q1 2023 or early Q2 2023.
- Selection of COVID-19 vaccine construct to be used in the UK human challenge study expected in Q4 2022.
- After determining which COVID-19 vaccine candidate to advance, Vaxart anticipates updating its plans for its India trials.
- Omicron Human Challenge Trial in the UK starting in 2H 2023 using selected vaccine construct.

Business Overview
Vxart Biosciences, Inc. was originally incorporated in California under the name West Coast Biologicals, Inc. in March 2004 and changed its name to Vaxart, Inc. (“Private Vaxart”) in July 2007.2
On February 13, 2018, Private Vaxart completed a reverse merger (the “Merger”) with Aviragen Therapeutics, Inc. (“Aviragen”), pursuant to which Private Vaxart survived as a wholly owned subsidiary of Aviragen. Under the terms of the Merger, Aviragen changed its name to Vaxart, Inc. and Private Vaxart changed its name to Vaxart Biosciences, Inc
Vaxart is a clinical-stage biotechnology company primarily focused on the development of oral recombinant vaccines based on its Vector-Adjuvant-Antigen Standardized Technology (VAAST) proprietary oral vaccine platform. The company's oral vaccines are designed to generate broad and durable immune responses that may protect against a wide range of infectious diseases and may be useful for the treatment of chronic viral infections and cancer. The company's investigational vaccines are administered using a room temperature-stable tablet, rather than by injection.
Vaxart is developing prophylactic vaccine candidates that target a range of infectious diseases, including SARS-CoV-2 (the virus that causes coronavirus disease 2019 (COVID-19)), norovirus (a widespread cause of acute gastro-intestinal enteritis), seasonal influenza and respiratory syncytial virus (RSV) (a common cause of respiratory tract infections). Vaxart has completed a Phase 1 clinical trial for its first SARS CoV-2 vaccine candidate, that commenced in October 2020; the study met its primary and secondary endpoints. A Phase 2 study with its second-generation SARS CoV-2 vaccine candidate commenced dosing in October 2021 and is currently ongoing. Three Phase 1 human studies for its norovirus vaccine candidate have been completed, including a study with a bivalent norovirus vaccine which, as the company disclosed in September 2019, met its primary and secondary endpoints. Additional Phase 1 studies with its norovirus vaccine are currently ongoing. The company's monovalent H1 influenza vaccine protected participants against H1 influenza infection in a Phase 2 challenge study, as published in 2020 (Lancet ID). In addition, Vaxart is in early development of its first therapeutic vaccine targeting cervical cancer and dysplasia caused by human papillomavirus (HPV).
Vaccines have been essential in eradicating or significantly reducing multiple devastating infectious diseases, including polio, smallpox, mumps, measles, diphtheria, hepatitis B, influenza, HPV and several others. According to a MarketsandMarkets research report titled Vaccines Market - Global Forecast to 2023, the global market for vaccines is expected to reach $50.42 billion by 2023 from $36.45 billion in 2018, at a compound annual growth rate of 6.7%.
Leased facilities as of December 31, 2021, are as follows:
| Location | Square Feet | Primary Use | Lease Terms |
South San Francisco and Burlingame, CA | 49,436 sq ft | Laboratory, manufacturing and office | Five leases expiring between July 2022 and March 2029 |
South San Francisco, CA | 24,544 sq ft | Manufacturing and office | One lease executed, commencing in 2022, expiring in March 2029 |




